1.11 Drawing Chemical Structures
In the structures we’ve been drawing until now, a line between atoms has represented the two electrons in a covalent bond. Drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. In condensed structures, carbon– hydrogen and carbon–carbon single bonds aren’t shown; instead, they’re understood. If a carbon has three hydrogens bonded to it, we write CH3; if a carbon has two hydrogens bonded to it, we write CH2; and so on. The compound called 2-methylbutane, for example, is written as follows:
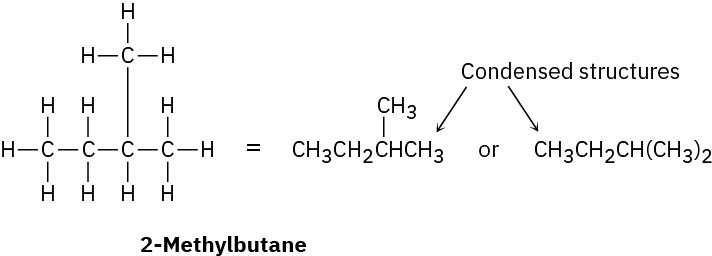 Note that the horizontal bonds between carbons aren’t shown in condensed structures— the CH3, CH2, and CH units are simply placed next to each other—but vertical carbon– carbon bonds like that of the first of the condensed structures drawn above is shown for clarity. Notice also in the second of the condensed structures that the two CH3 units attached to the CH carbon are grouped together as (CH3)2.
Note that the horizontal bonds between carbons aren’t shown in condensed structures— the CH3, CH2, and CH units are simply placed next to each other—but vertical carbon– carbon bonds like that of the first of the condensed structures drawn above is shown for clarity. Notice also in the second of the condensed structures that the two CH3 units attached to the CH carbon are grouped together as (CH3)2.
Even simpler than condensed structures are skeletal structures such as those shown in Table 1.3. The rules for drawing skeletal structures are straightforward.
RULE 1
Carbon atoms aren’t usually shown. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity.
RULE 2
Hydrogen atoms bonded to carbon aren’t shown. Because carbon always has a valence of 4, we mentally supply the correct number of hydrogen atoms for each carbon.
RULE 3
Atoms other than carbon and hydrogen are shown.
One further comment: Although such groupings as –CH3, –OH, and –NH2 are usually written with the C, O, or N atom first and the H atom second, the order of writing is sometimes inverted to H3C–, HO–, and H2N– if needed to make the bonding connections clearer. Larger units such as –CH2CH3 are not inverted, though; we don’t write H3CH2C– because it would be confusing. There are, however, no well-defined rules that cover all cases; it’s largely a matter of preference.
Table 1.3 Line-bond and Skeletal Structures for Some Compounds
|
Line-bond structure |
Skeletal structure |
|
|
Isoprene, C5H8 |
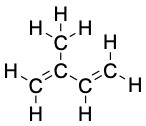 |
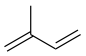 |
|
Methylcyclohexane, C7H14 |
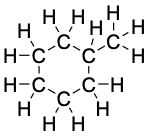 |
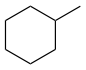 |
|
Phenol, C6H6O |
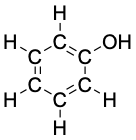 |
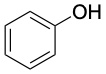 |
Worked Example 1.4: Interpreting a Line-Bond Structure
Carvone, a substance responsible for the odor of spearmint, has the following structure. Tell how many hydrogens are bonded to each carbon, and give the molecular formula of carvone.
Strategy
The end of a line represents a carbon atom with 3 hydrogens, CH3; a two-way intersection is a carbon atom with 2 hydrogens, CH2; a three-way intersection is a carbon atom with 1 hydrogen, CH; and a four-way intersection is a carbon atom with no attached hydrogens.
Solution
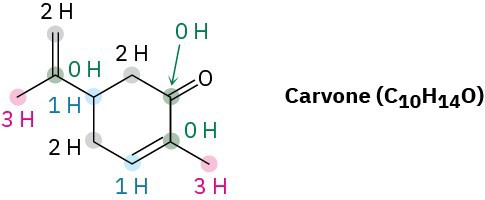
Problem 1.15
How many hydrogens are bonded to each carbon in the following compounds, and what is the molecular formula of each substance?
(a)
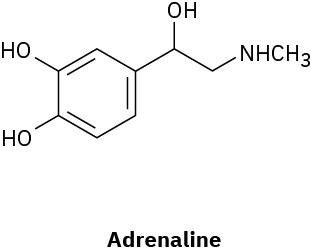
(b)
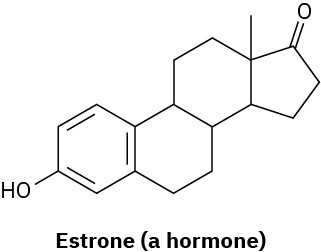
Problem 1.16
Propose skeletal structures for compounds that satisfy the following molecular formulas: There is more than one possibility in each case.
(a) C5H12
(b) C2H7N
(c) C3H6O
(d) C4H9Cl
Problem 1.17
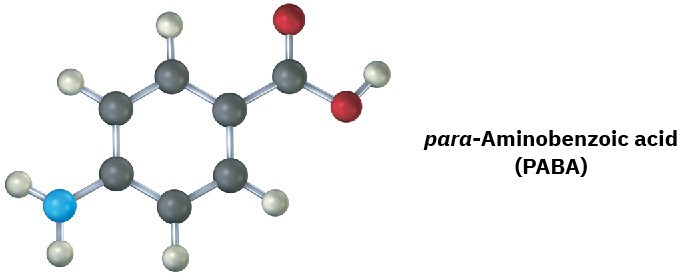
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds, and draw a skeletal structure (black = C, red = O, blue = N, gray = H).