5.2 Carbocation Structure and Stability
To understand why Markovnikov’s rule works, we need to learn more about the structure and stability of carbocations and about the general nature of reactions and transition states. The first point to explore involves structure.
A great deal of experimental evidence has shown that carbocations are planar. The trivalent carbon is sp2-hybridized, and the three substituents are oriented toward the corners of an equilateral triangle, as indicated in Figure 5.4. Because there are only six valence electrons on carbon and all six are used in the three σ bonds, the p orbital extending above and below the plane is unoccupied.
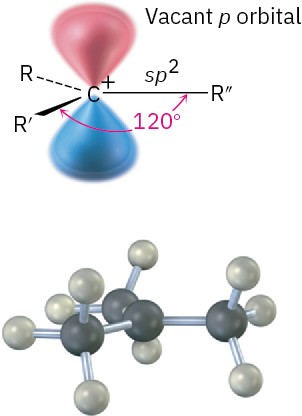 Figure 5.4 The structure of a carbocation. The trivalent carbon is sp2-hybridized and has a vacant p orbital perpendicular to the plane of the carbon and three attached groups.
Figure 5.4 The structure of a carbocation. The trivalent carbon is sp2-hybridized and has a vacant p orbital perpendicular to the plane of the carbon and three attached groups.
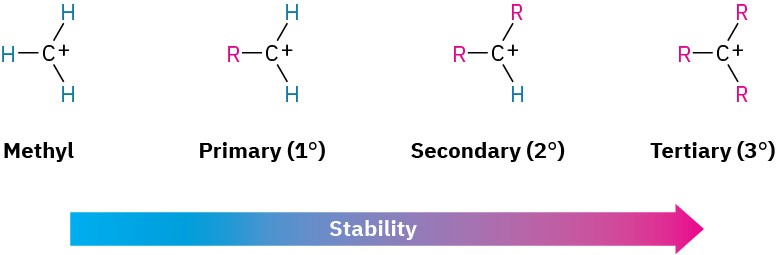
The second point to explore involves carbocation stability. 2-Methylpropene might react with H+ to form a carbocation having three alkyl substituents (a tertiary ion, 3°), or it might react to form a carbocation having one alkyl substituent (a primary ion, 1°). Since the tertiary alkyl chloride, 2-chloro-2-methylpropane, is the only product observed, formation of the tertiary cation is evidently favored over formation of the primary cation.
Thermodynamic measurements show that, indeed, the stability of carbocations increases with increasing substitution so that the stability order is tertiary > secondary > primary > methyl.
Why are more highly substituted carbocations more stable than less highly substituted ones? There are at least two reasons. Part of the answer has to do with inductive effects, and part has to do with hyperconjugation. Inductive effects, discussed in Section 1.12 in connection with polar covalent bonds, result from the shifting of electrons in a σ bond in response to the electronegativity of nearby atoms. In the present instance, electrons from a relatively larger and more polarizable alkyl group can shift toward a neighboring positive charge more easily than the electron from a hydrogen. Thus, the more alkyl groups attached to the positively charged carbon, the more electron density shifts toward the charge and the more inductive stabilization of the cation occurs (Figure 5.5).
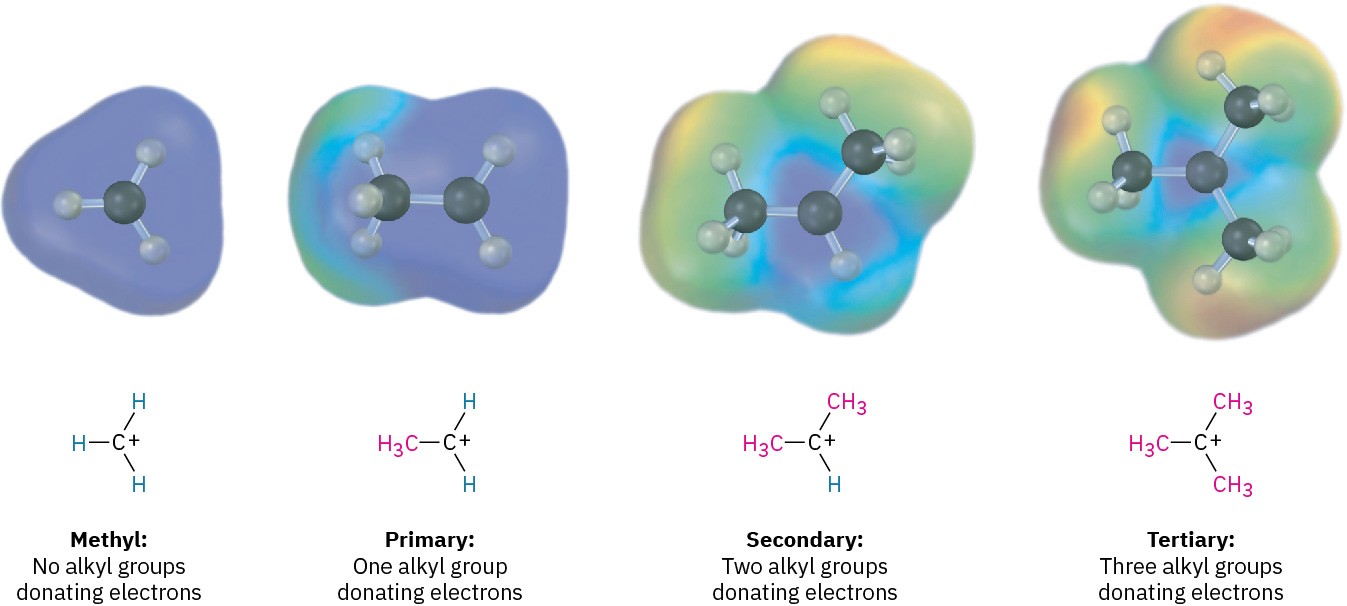
Figure 5.5 A comparison of inductive stabilization for methyl, primary, secondary, and tertiary carbocations. The more alkyl groups that are bonded to the positively charged carbon, the more electron density shifts toward the charge, making the charged carbon less electron-poor (blue in electrostatic potential maps).
Problem 5.3
Show the structures of the carbocation intermediates you would expect in the following reactions:
(a)
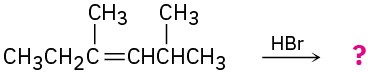
(b)