Additional Problems 5
Visualizing Chemistry
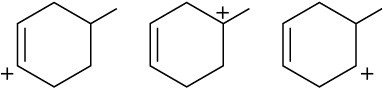
Problem 5.17
The following alkyl bromide can be made by HBr addition to three different alkenes. Show their structures.
Problem 5.18
Name the following alkynes, and predict the products of their reaction with (1) H2 in the presence of a Lindlar catalyst and (2) H3O+ in the presence of HgSO4:
(a)
(b)
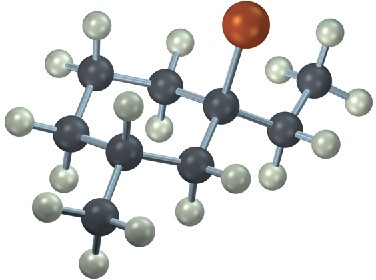
Problem 5.19
From what alkyne might each of the following substance have been made? (Green = Cl.)
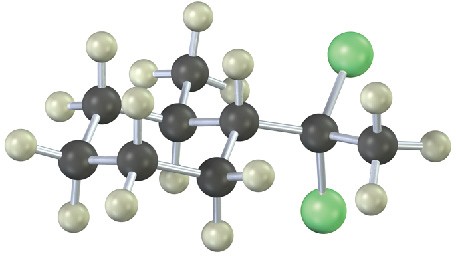
Problem 5.20
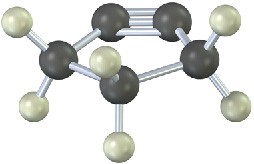
The following cycloalkyne is too unstable to exist. Explain.
Reactions of Alkenes
Problem 5.21
Suggest structures for alkenes that give the following reaction products. There may be more than one answer for some cases.
(a)
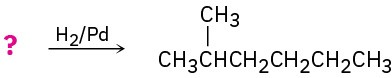
(b)
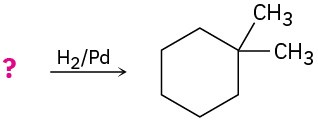
(c)
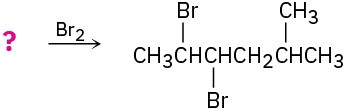
(d)
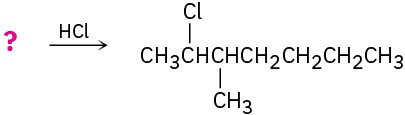
Problem 5.22
Show the structures of all possible adducts of the following diene with 1 equivalent of HCl:
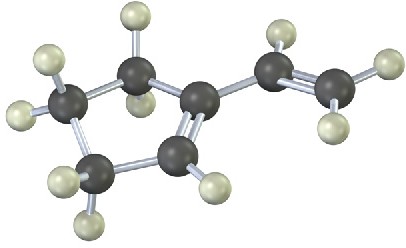
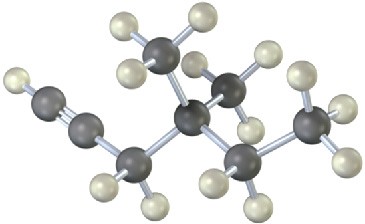
Problem 5.23
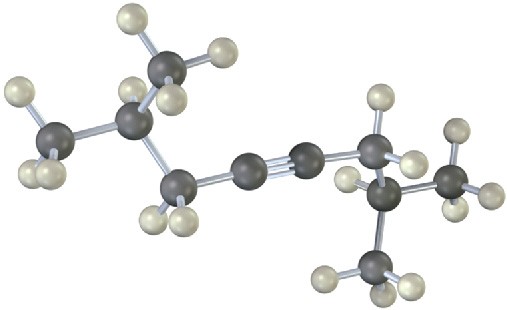
The following model is that of an allylic carbocation intermediate formed by protonation of a conjugated diene with HBr. Show the structure of the diene and the structures of the final reaction products.
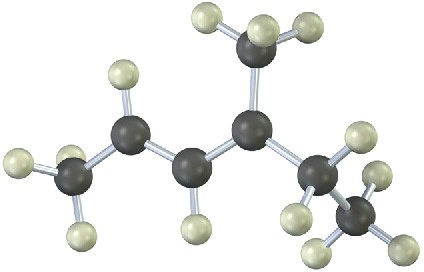
Problem 5.24
Predict the major product(s) from the addition of 1 equivalent of HX for each of the following reactions.
(a)
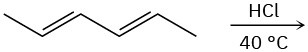
(b)
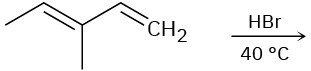
(c)
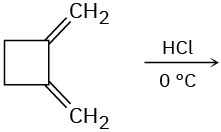
Problem 5.25
Propose a structure for a conjugated diene that gives the same product from both 1,2 and 1,4-addition of HBr.
Mechanism Problems
Problem 5.26
Predict the major product and show the complete mechanism for each of the following electrophilic addition reactions.
(a)
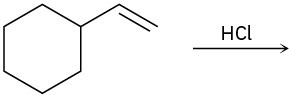
(b)
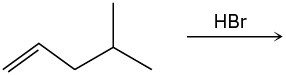
(c)
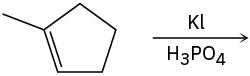
Problem 5.27
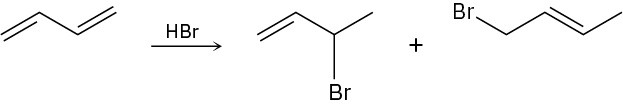
When 1,3-butadiene reacts with 1 mol of HBr, two isolable products result. Propose mechanisms for both.
Problem 5.28
Predict the products for the following reactions, showing the complete mechanism and appropriate stereochemistry:
(a)
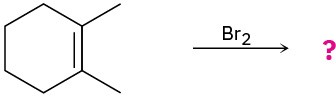
(b)
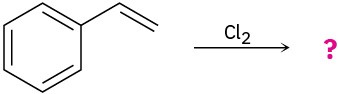
(c)
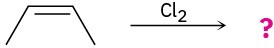
Problem 5.29
meta-Chlorobenzoic acid is not the only peroxyacid capable of epoxide formation. For each reaction below, predict the products.
(a)
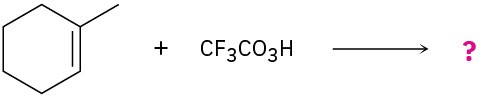
(b)
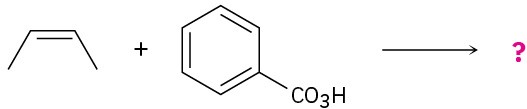
Problem 5.30
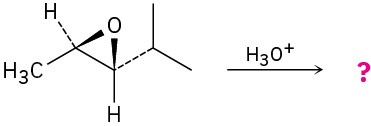
Give the mechanism and products for the following acid-catalyzed epoxide-opening reactions, including appropriate stereochemistry.
(a)
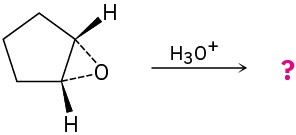
(b)
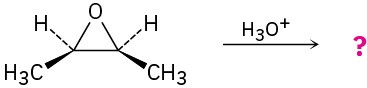
(c)
Carbocations and Electrophilic Addition Reactions
Problem 5.31
Rank the following carbocations according to their increasing stability.
(a)
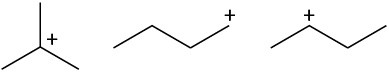
(b)
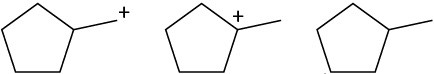
(c)
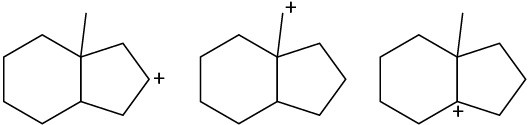
Problem 5.32
Predict the major product in each of the following reactions:
(a)
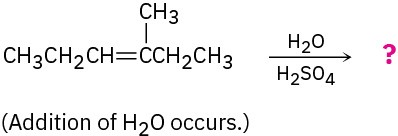
(b)
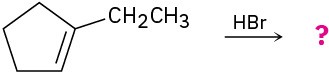
Problem 5.33
Alkenes can be converted into alcohols by acid-catalyzed addition of water. Assuming that Markovnikov’s rule is valid, predict the major alcohol product from each of the following alkenes.
(a)
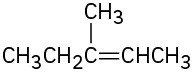
(b)
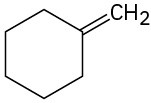
(c)
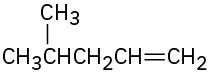
Polymers
Problem 5.34
Plexiglas, a clear plastic used to make many molded articles, is made by polymerization of methyl methacrylate. Draw a representative segment of Plexiglas.
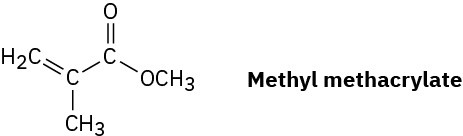
Problem 5.35
Poly(vinyl pyrrolidone), prepared from N-vinylpyrrolidone, is used both in cosmetics and as a component of a synthetic substitute for blood. Draw a representative segment of the polymer.
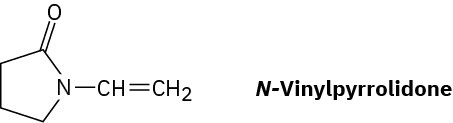
Problem 5.36
When a single alkene monomer, such as ethylene, is polymerized, the product is a homopolymer. If a mixture of two alkene monomers is polymerized, however, a copolymer often results. The following structure represents a segment of a copolymer called Saran. What two monomers were copolymerized to make Saran?
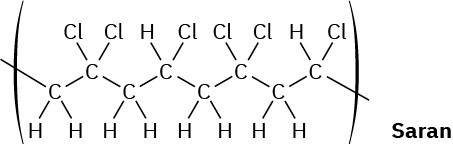
Reactions of Alkynes
Problem 5.37
We saw in this chapter that alkynes undergo many of the same reactions that alkenes do. What product might you expect from each of the following reactions?
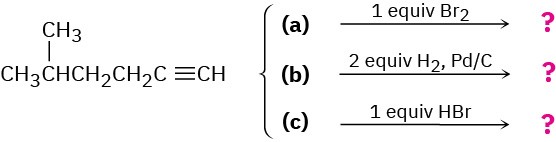
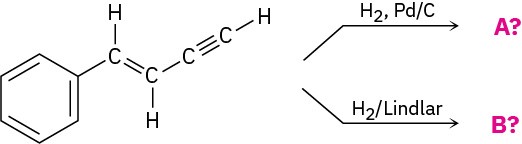
Problem 5.38
Predict the products of the following reactions:
Problem 5.39
Predict the products from reaction of 1-hexyne with the following reagents:
(a) 1 equiv HBr
(b) 1 equiv Cl2
(c) H2, Lindlar catalyst
(d) NaNH2 in NH3, then CH3Br
(e) H2O, H2SO4, HgSO4
(f) 2 equiv HCl
Problem 5.40
Predict the products from reaction of 5-decyne with the following reagents:
(a) H2, Lindlar catalyst
(b) 1 equiv Br2
(c) H2O, H2SO4, HgSO4
(d) Excess H2, Pd/C catalyst
Problem 5.41
Predict the products from reaction of 2-hexyne with the following reagents:
(a) 2 equiv Br2
(b) 1 equiv HBr
(c) Excess HBr
(d) H2O, H2SO4, HgSO4
Problem 5.42
How would you carry out the following reactions?
(a)
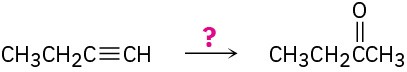
(b)
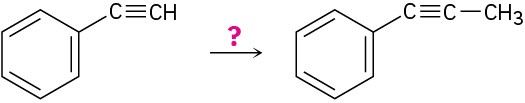
(c)
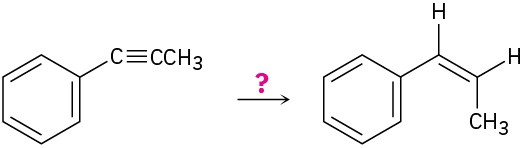
Problem 5.43
Arrange the following carbocations in order of increasing stability.
(a)
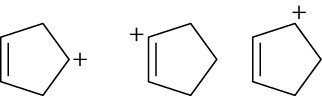
(b)
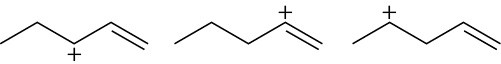
(c)