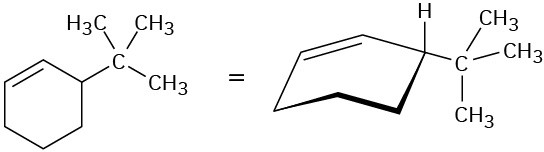
5.5 Reduction of Alkenes: Hydrogenation
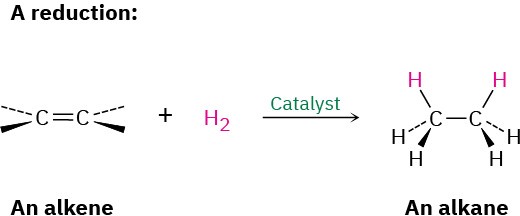
Alkenes react with H2 in the presence of a metal catalyst such as palladium or platinum to yield the corresponding saturated alkanes. We describe the result by saying that the double bond has been hydrogenated, or reduced. Note that the word reduction is used somewhat differently in organic chemistry from what you might have learned previously. In general chemistry, a reduction is defined as the gain of one or more electrons by an atom. In organic chemistry, however, a reduction is a reaction that results in a gain of electron density for carbon, caused either by bond formation between carbon and a less electronegative atom—usually hydrogen—or by bond-breaking between carbon and a more electronegative atom—usually oxygen, nitrogen, or a halogen. We’ll explore this topic in more detail in Section 6.5.

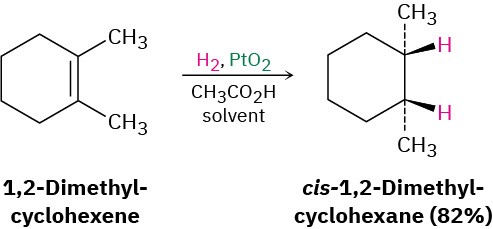
 Platinum and palladium are the most common laboratory catalysts for alkene hydrogenations. Palladium is normally used as a very fine powder “supported” on an inert material such as charcoal (Pd/C) to maximize surface area. Platinum is normally used as PtO2, a reagent known as Adams’ catalyst after its discoverer, Roger Adams at the University of Illinois.
Platinum and palladium are the most common laboratory catalysts for alkene hydrogenations. Palladium is normally used as a very fine powder “supported” on an inert material such as charcoal (Pd/C) to maximize surface area. Platinum is normally used as PtO2, a reagent known as Adams’ catalyst after its discoverer, Roger Adams at the University of Illinois.
Catalytic hydrogenation, unlike most other organic reactions, is a heterogeneous process rather than a homogeneous one. That is, the hydrogenation reaction does not occur in a homogeneous solution but instead takes place on the surface of solid catalyst particles. Hydrogenation usually occurs with syn stereochemistry: both hydrogens add to the double bond from the same face.
 As shown in Figure 5.7, hydrogenation begins with adsorption of H2 onto the catalyst surface. Complexation between catalyst and alkene then occurs as a vacant orbital on the metal interacts with the filled alkene π orbital on the alkene. In the final steps, hydrogen is inserted into the double bond and the saturated product diffuses away from the catalyst. The stereochemistry of hydrogenation is syn because both hydrogens add to the double bond from the same catalyst surface.
As shown in Figure 5.7, hydrogenation begins with adsorption of H2 onto the catalyst surface. Complexation between catalyst and alkene then occurs as a vacant orbital on the metal interacts with the filled alkene π orbital on the alkene. In the final steps, hydrogen is inserted into the double bond and the saturated product diffuses away from the catalyst. The stereochemistry of hydrogenation is syn because both hydrogens add to the double bond from the same catalyst surface.
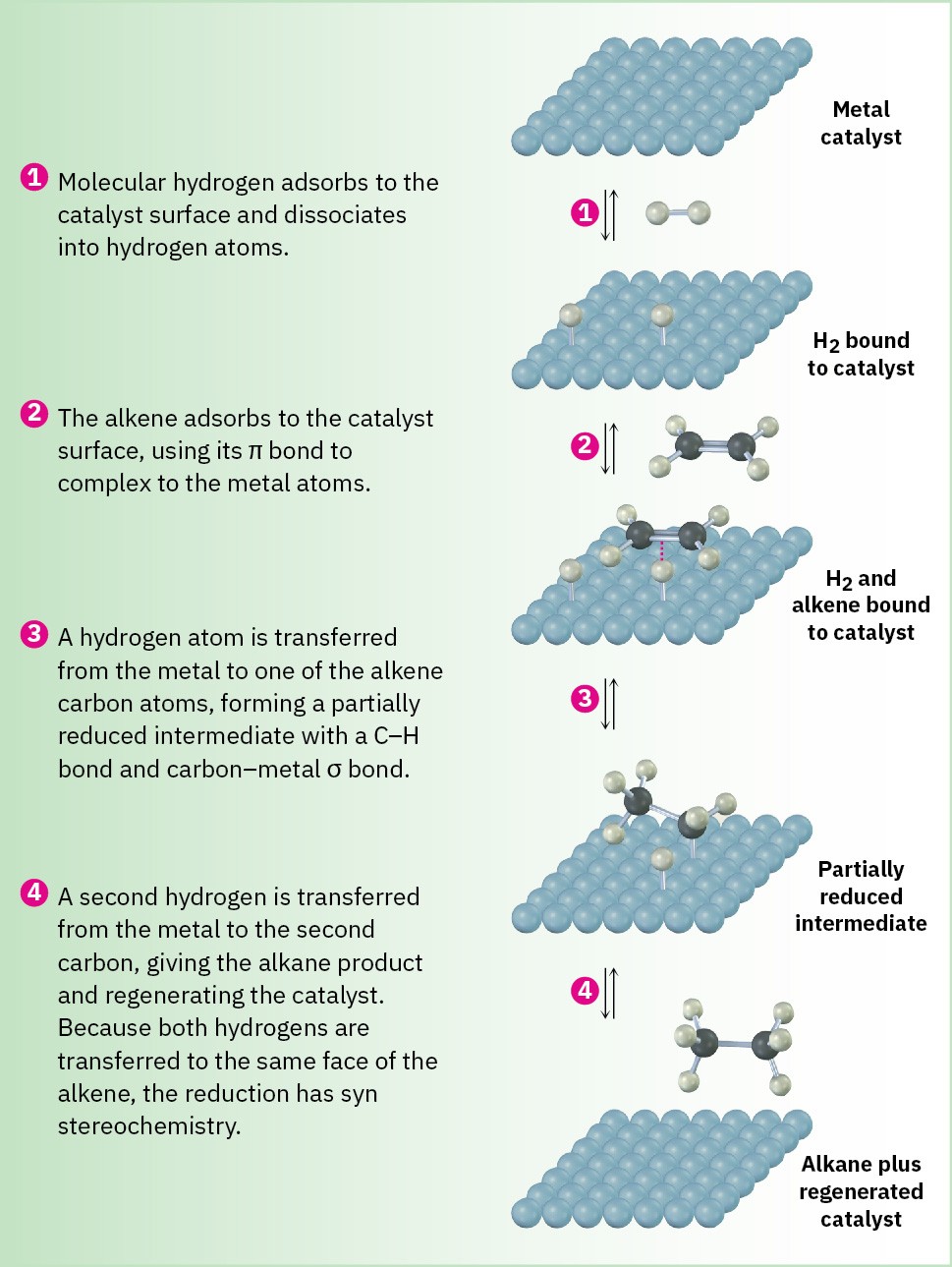
Figure 5.7 MECHANISM: Mechanism of alkene hydrogenation. The reaction takes place with syn stereochemistry on the surface of insoluble catalyst particles.
In addition to its usefulness in the laboratory, catalytic hydrogenation is also important in the food industry, where unsaturated vegetable oils are reduced on a large scale to produce the saturated fats used in margarine and cooking products (Figure 5.8). Vegetable oils are triesters of glycerol, HOCH2CH(OH)CH2OH, with three long-chain carboxylic acids called fatty acids. The fatty acids are generally polyunsaturated, and their double bonds have cis stereochemistry. Complete hydrogenation yields the corresponding saturated fatty acids, but incomplete hydrogenation often results in partial cis–trans isomerization of a remaining double bond. When eaten and digested, the free trans fatty acids are released, raising blood cholesterol levels and potentially contributing to coronary problems.
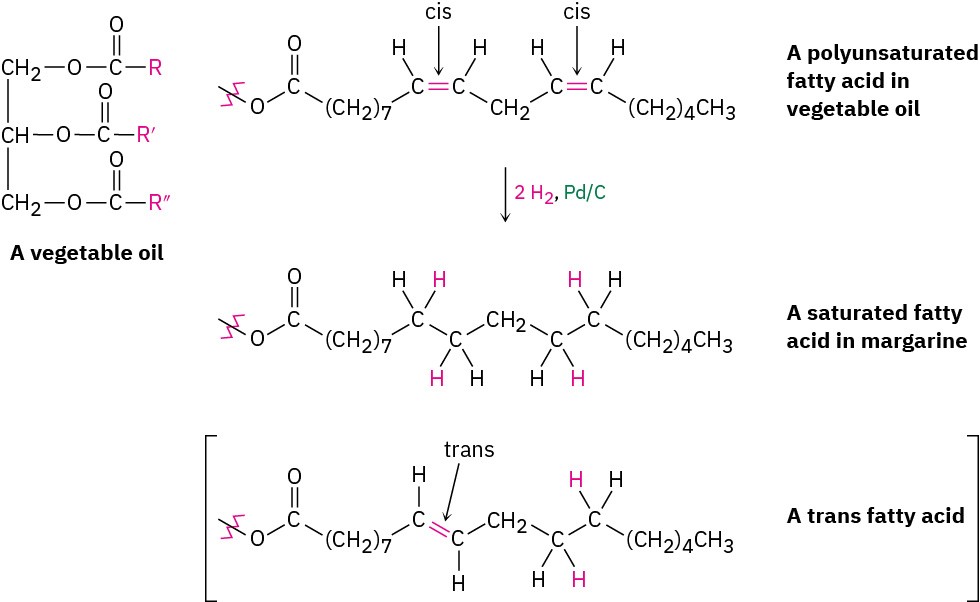 Figure 5.8 Catalytic hydrogenation of polyunsaturated fats leads primarily to saturated products, along with a small amount of isomerized trans fats.
Figure 5.8 Catalytic hydrogenation of polyunsaturated fats leads primarily to saturated products, along with a small amount of isomerized trans fats.
Problem 5.6
What product would you obtain from catalytic hydrogenation of the following alkenes?
(a)
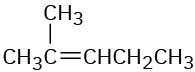
(b)
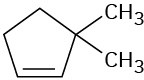
(c)