6.2 Preparing Alkyl Halides from Alkanes: Radical Halogenation
As we saw previously, simple alkyl halides can sometimes be prepared by the radical reaction of an alkane with Cl2 or Br2 in the presence of ultraviolet light. The detailed mechanism is shown in Figure 6.2 for chlorination.
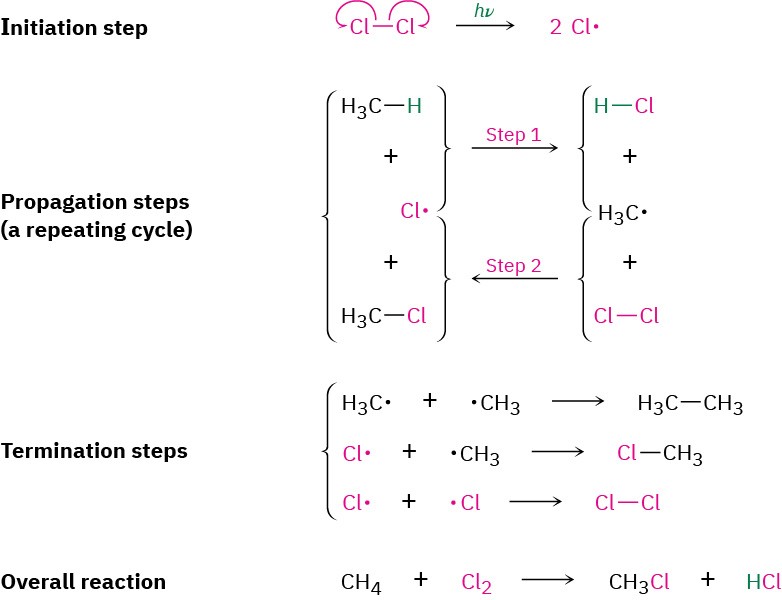 Figure 6.2 Mechanism of the radical chlorination of methane.
Figure 6.2 Mechanism of the radical chlorination of methane.
Three kinds of steps are required in radical substitution reactions: initiation, propagation, and termination. The propagation steps are a repeating cycle, with Cl· a reactant in step 1 and a product in step 2, and with ·CH3 a product in step 1 and a reactant in step 2. The symbol hν shown in the initiation step is the standard way of indicating irradiation with light (ν is the lowercase Greek letter nu).
Radical substitution reactions require three kinds of steps: initiation, propagation, and termination. Once an initiation step has started the process by producing radicals, the reaction continues in a self-sustaining cycle. The cycle requires two repeating propagation steps in which a radical, the halogen, and the alkane yield alkyl halide product plus more radical to carry on the chain. The chain is occasionally terminated by the combination of two radicals.
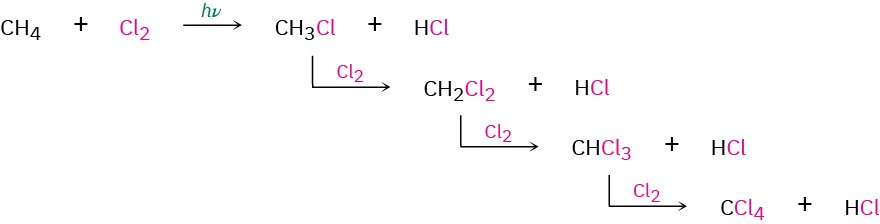
Unfortunately, alkane halogenation is a poor synthetic method for preparing alkyl halides because mixtures of products invariably result. For example, chlorination of methane does not stop cleanly at the monochlorinated stage but continues to give a mixture of dichloro, trichloro, and even tetrachloro products.