1 Why This Chapter?
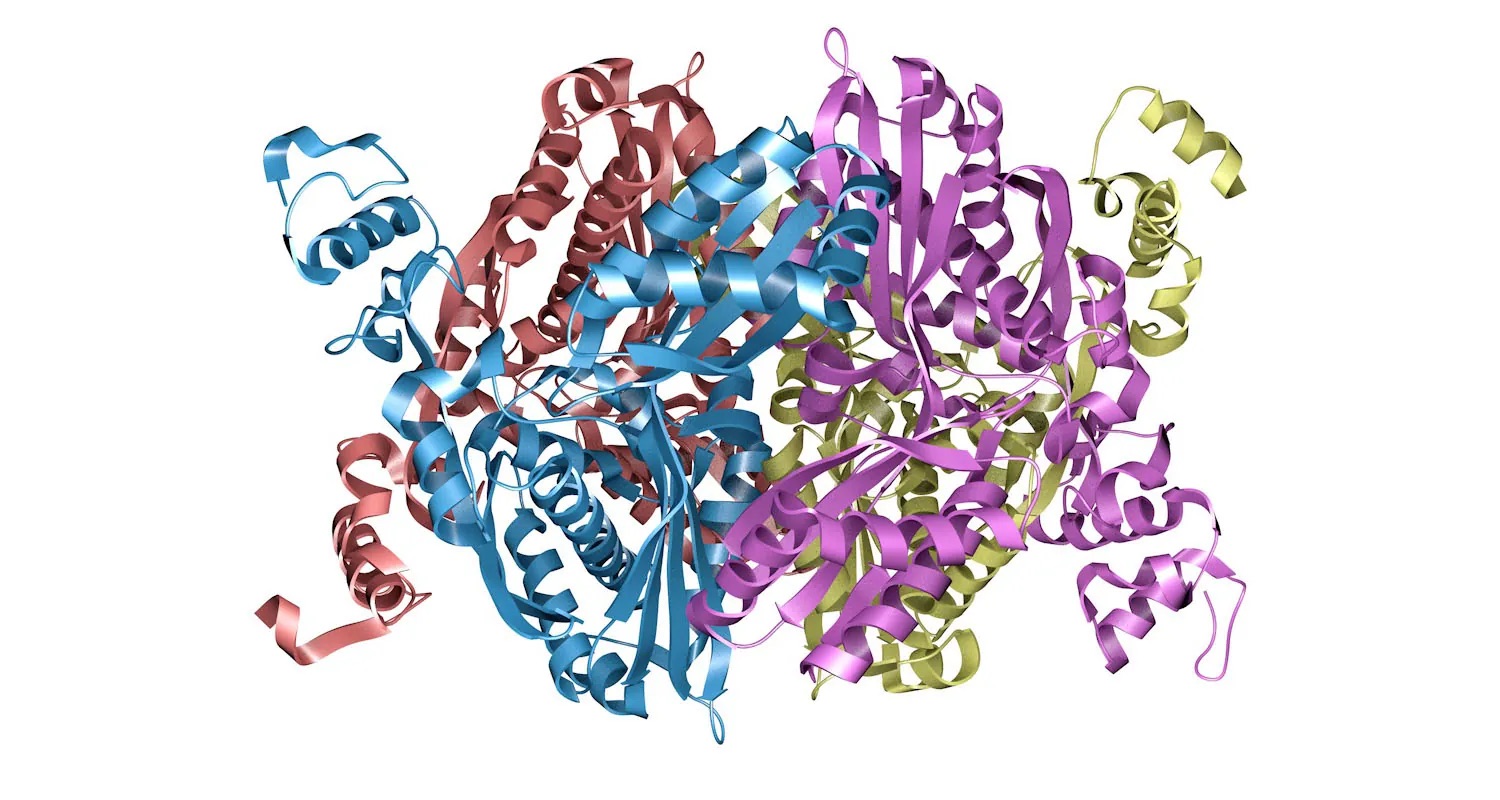
Figure 1.1 The enzyme HMG–CoA reductase, shown here as a so-called ribbon model, catalyzes a crucial step in the body’s synthesis of cholesterol. Understanding how this enzyme functions has led to the development of drugs credited with saving millions of lives. (credit: image from the RCSB PDB (rcsb.org) of PBD ID 1HW9 (E.S. Istvan, J. Deisenhofer) (2001) Structural mechanism for statin inhibition of HMG-CoA reductase Science 292: 1160–1164/RCSB PDB, CC BY 1.0)
We’ll ease into the study of organic chemistry by first reviewing some ideas about atoms, bonds, and molecular geometry that you may recall from your general chemistry course. Much of the material in this chapter and the next is likely to be familiar to you, but it’s nevertheless a good idea to make sure you understand it before moving on.
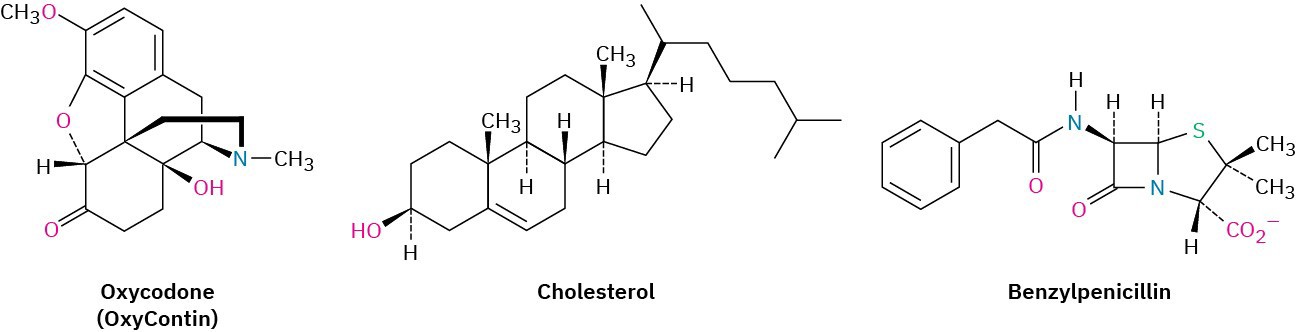
What is organic chemistry, and why should you study it? The answers to these questions are all around you. Every living organism is made of organic chemicals. The proteins that make up your hair, skin, and muscles; the DNA that controls your genetic heritage; the foods that nourish you; and the medicines that heal you are all organic chemicals. Anyone with a curiosity about life and living things, and anyone who wants to be a part of the remarkable advances taking place in medicine and the biological sciences, must first understand organic chemistry. Look at the following drawings for instance, which show the chemical structures of some molecules whose names might be familiar to you. Although the drawings may appear unintelligible at this point, don’t worry. They’ll make perfectly good sense before long, and you’ll soon be drawing similar structures for any substance you’re interested in.
 Historically, the term organic chemistry dates to the mid-1700s, when it was used to mean the chemistry of substances found in living organisms. Little was known about chemistry at that time, and the behavior of the “organic” substances isolated from plants and animals seemed different from that of the “inorganic” substances found in minerals. Organic compounds were generally low-melting solids and were usually more difficult to isolate, purify, and work with than high-melting inorganic compounds.
Historically, the term organic chemistry dates to the mid-1700s, when it was used to mean the chemistry of substances found in living organisms. Little was known about chemistry at that time, and the behavior of the “organic” substances isolated from plants and animals seemed different from that of the “inorganic” substances found in minerals. Organic compounds were generally low-melting solids and were usually more difficult to isolate, purify, and work with than high-melting inorganic compounds.
By the mid-1800s, however, it was clear that there was no fundamental difference between organic and inorganic compounds. The only distinguishing characteristic of organic compounds is that all contain the element carbon.
Organic chemistry, then, is the study of carbon compounds. But why is carbon special? Why, of the more than 197 million presently known chemical compounds, do almost all of them contain carbon? The answers to these questions come from carbon’s electronic structure and its consequent position in the periodic table (Figure 1.2). As a group 4A element, carbon can share four valence electrons and form four strong covalent bonds. Furthermore, carbon atoms can bond to one another, forming long chains and rings.
Carbon, alone of all elements, is able to form an immense diversity of compounds, from the simple methane, with one carbon atom, to the staggeringly complex DNA, which can have more than 100 million carbons.
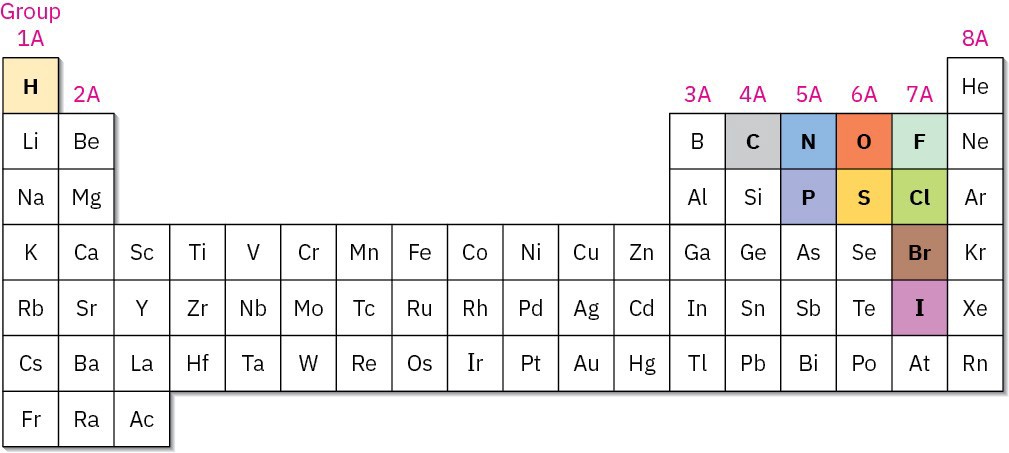
Figure 1.2 Carbon, hydrogen, and other elements commonly found in organic compounds are shown in the colors typically used to represent them.
Not all carbon compounds are derived from living organisms, however. Modern chemists have developed a remarkably sophisticated ability to design and synthesize new organic compounds in the laboratory—medicines, dyes, polymers, and a host of other substances. Organic chemistry touches the lives of everyone. Its study can be a fascinating undertaking.

Figure 1.3 The opium poppy is the source of morphine, one of the first “vegetable alkali,” or alkaloids, to be isolated. (credit: modification of work “Papaver somniferum” by Liz West/Flickr, CC BY 2.0)
Understanding organic chemistry means knowing not just what happens but also why and how it happens at the molecular level. In this chapter, we’ll look at some of the ways that chemists describe and account for chemical reactivity, thereby providing a foundation to understand the specific reactions discussed in subsequent chapters. Topics such as bond polarity, the acid–base behavior of molecules, and hydrogen-bonding are a particularly important part of that foundation.
We will see how covalent bonds between atoms are described, and we will look at the valence bond model, which uses hybrid orbitals to account for the observed shapes of organic molecules. Before going on to a systematic study of organic chemistry, however, we still need to review a few fundamental topics. In particular, we need to look more closely at how electrons are distributed in covalent bonds and at some of the consequences that arise when the electrons in a bond are not shared equally between atoms.

