7.2 The SN2 Reaction
In almost all chemical reactions, there is a direct relationship between the rate at which the reaction occurs and the concentrations of the reactants. When we measure this relationship, we measure the kinetics of the reaction. For example, let’s look at the kinetics of a simple nucleophilic substitution—the reaction of CH3Br with OH– to yield CH3OH plus Br–.
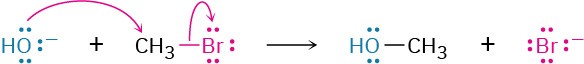 With a given temperature, solvent, and concentration of reactants, the substitution occurs at a certain rate. If we double the concentration of OH–, the frequency of encounters between reaction partners doubles and we find that the reaction rate also doubles.
With a given temperature, solvent, and concentration of reactants, the substitution occurs at a certain rate. If we double the concentration of OH–, the frequency of encounters between reaction partners doubles and we find that the reaction rate also doubles.
Similarly, if we double the concentration of CH3Br, the reaction rate again doubles. We call such a reaction, in which the rate is linearly dependent on the concentrations of two species, a second-order reaction. Mathematically, we can express this second-order dependence of the nucleophilic substitution reaction by setting up a rate equation. As either [RX] or [–OH] changes, the rate of the reaction changes proportionately.
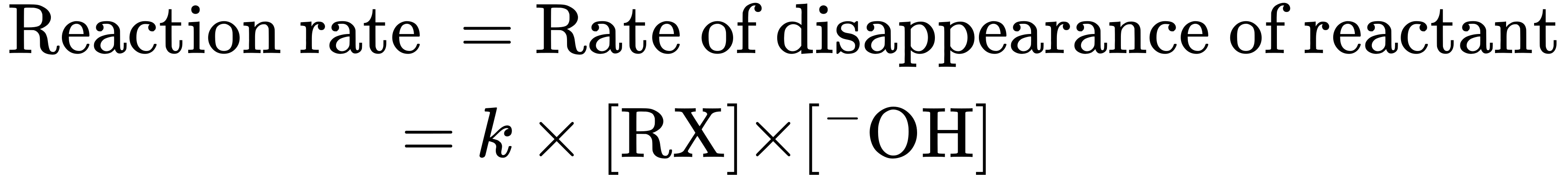
where
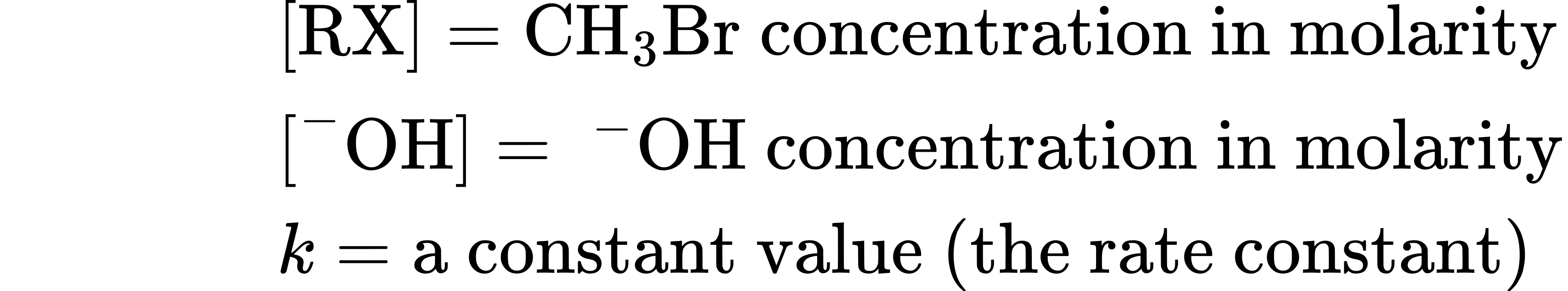
A mechanism that accounts for both the inversion of configuration and the second-order kinetics that are observed with nucleophilic substitution reactions was suggested in 1937 by the British chemists E. D. Hughes and Christopher Ingold, who formulated what they called the SN2 reaction—short for substitution, nucleophilic, bimolecular. (Bimolecular means that two molecules, nucleophile and alkyl halide, take part in the step whose kinetics are measured.)
The essential feature of the SN2 mechanism is that it takes place in a single step, without intermediates, when the incoming nucleophile reacts with the alkyl halide or tosylate (the substrate) from a direction opposite the group that is displaced (the leaving group). As the nucleophile comes in on one side of the substrate and bonds to the carbon, the halide or tosylate departs from the other side, thereby inverting the stereochemical configuration. The process is shown in Figure 7.3 for the reaction of (S)-2-bromobutane with HO– to give (R)-2-butanol.
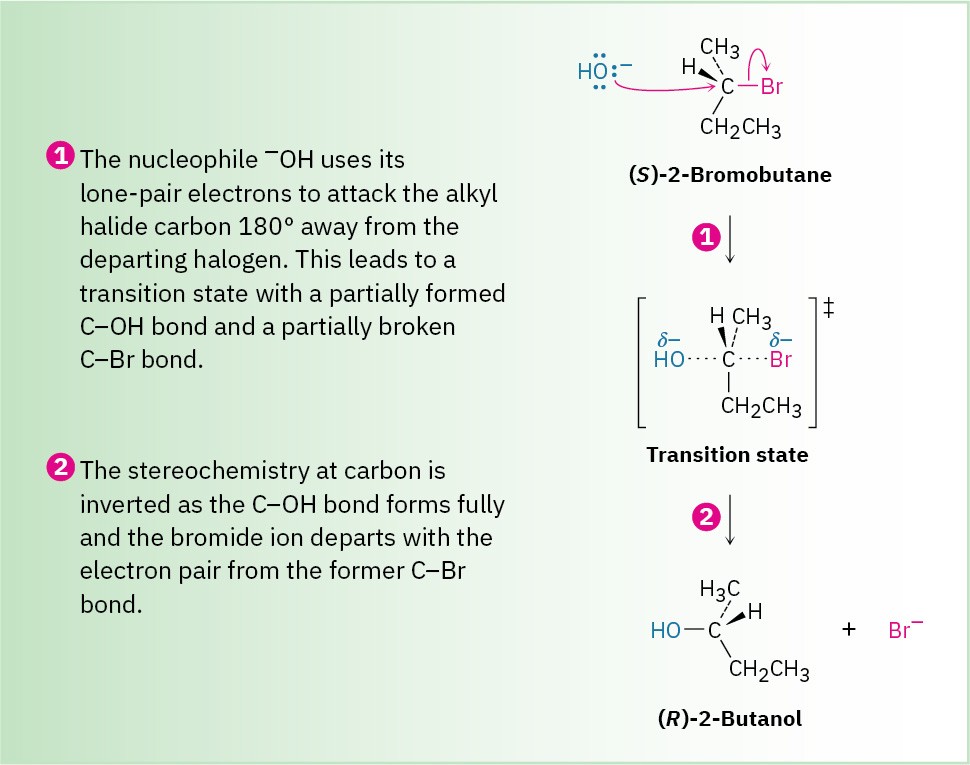
Figure 7.3 MECHANISM The mechanism of the SN2 reaction. The reaction takes place in a single step when the incoming nucleophile approaches from a direction 180° away from the leaving halide ion, thereby inverting the stereochemistry at carbon.
As shown in Figure 7.3, the SN2 reaction occurs when an electron pair on the nucleophile Nu:– forces out the group X:–, which takes with it the electron pair from the former C–X bond. This occurs through a transition state in which the new Nu–C bond is partially formed at the same time that the old C–X bond is partially broken and in which the negative charge is shared by both the incoming nucleophile and the outgoing halide ion. The transition state for this inversion has the remaining three bonds to carbon in a planar arrangement (Figure 7.4).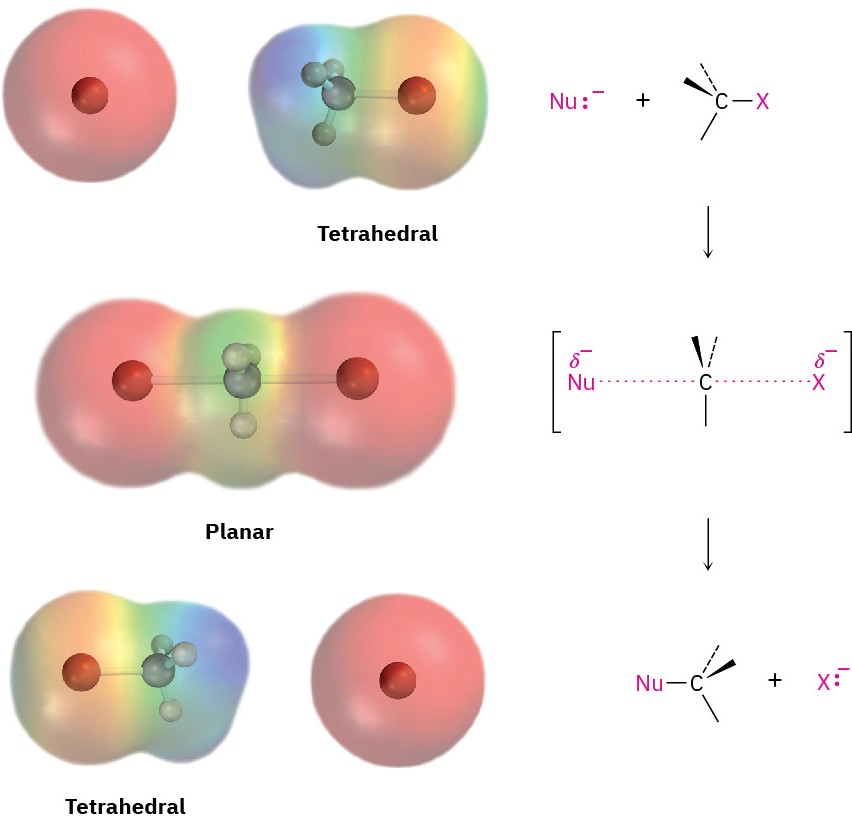 Figure 7.4 The transition state of an SN2 reaction has a planar arrangement of the carbon atom and the remaining three groups. Electrostatic potential maps show that negative charge is delocalized in the transition state.
Figure 7.4 The transition state of an SN2 reaction has a planar arrangement of the carbon atom and the remaining three groups. Electrostatic potential maps show that negative charge is delocalized in the transition state.
The mechanism proposed by Hughes and Ingold is fully consistent with experimental results, explaining both stereochemical and kinetic data. Thus, the requirement for a backside approach of the entering nucleophile (180° away from the departing X group) causes the stereochemistry of the substrate to invert, much like an umbrella turning inside- out in the wind. The Hughes–Ingold mechanism also explains why second-order kinetics are observed: the SN2 reaction occurs in a single step that involves both alkyl halide and nucleophile. Two molecules are involved in the step whose rate is measured.
Problem 7.2
What product would you expect to obtain from SN2 reaction of OH– with (R)-2-bromobutane? Show the stereochemistry of both the reactant and product.
Problem 7.3
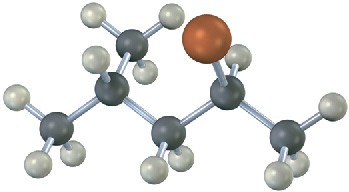
Assign configuration to the following substance, and draw the structure of the product that would result from nucleophilic substitution reaction with HS– (reddish brown = Br):