7.3 Characteristics of the SN2 Reaction
Now that we know how SN2 reactions occur, we need to see how they can be used and what variables affect them. Some SN2 reactions are fast, and some are slow; some take place in high yield and others in low yield. Understanding the factors involved can be of tremendous value.
Steric Effects in the SN2 Reaction
The first SN2 reaction variable to look at is the structure of the substrate. Because the SN2 transition state involves partial bond formation between the incoming nucleophile and the alkyl halide carbon atom, it seems reasonable that a hindered, bulky substrate should prevent easy approach of the nucleophile, making bond formation difficult. In other words, the transition state for reaction of a sterically hindered substrate, whose carbon atom is “shielded” from the approach of the incoming nucleophile, is higher in energy and forms more slowly than the corresponding transition state for a less hindered substrate (Figure 7.5).
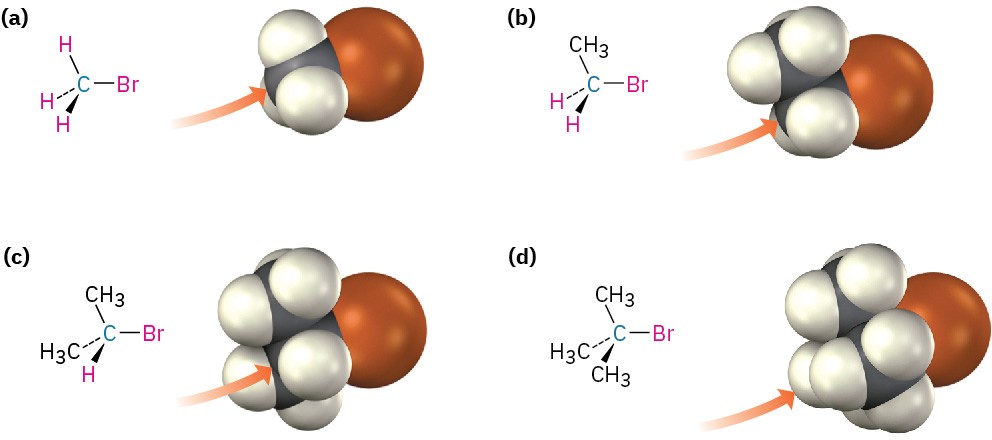
Figure 7.5 Steric hindrance to the SN2 reaction. As the models indicate, the carbon atom in (a) bromomethane is readily accessible, resulting in a fast SN2 reaction. The carbon atoms in (b) bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2-methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions.
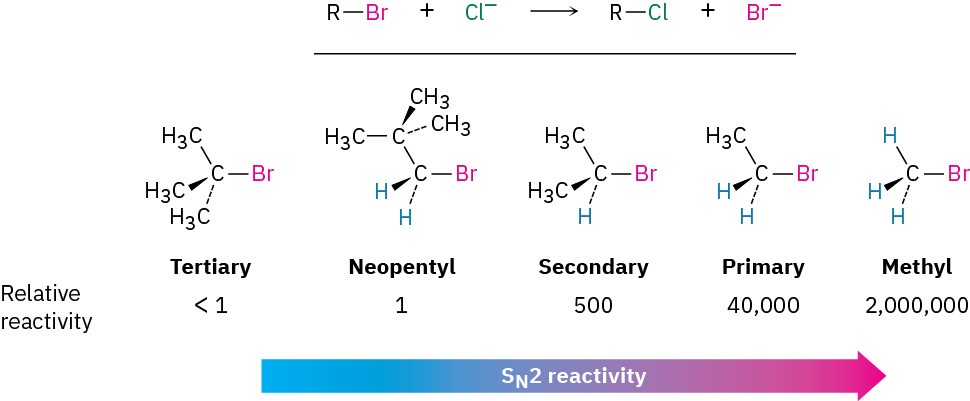
As Figure 7.5 shows, the difficulty of nucleophile approach increases as the three substituents bonded to the halo-substituted carbon atom increase in size. Methyl halides are by far the most reactive substrates in SN2 reactions, followed by primary alkyl halides such as ethyl and propyl. Alkyl branching at the reacting center, as in isopropyl halides (2°), slows the reaction greatly, and further branching, as in tert-butyl halides (3°), effectively halts the reaction. Even branching one carbon away from the reacting center, as in 2,2-dimethylpropyl (neopentyl) halides, greatly hinders nucleophilic displacement. As a result, SN2 reactions occur only at relatively unhindered sites and are normally useful only with methyl halides, primary halides, and a few simple secondary halides. Relative reactivities for some different substrates are as follows:
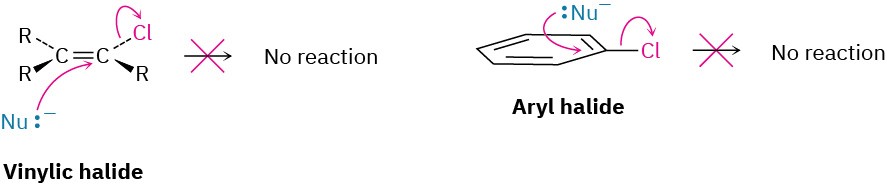
 Vinylic halides (R2C═CRX) and aryl halides are not shown on this reactivity list because they are unreactive toward SN2 displacement. This lack of reactivity is due to steric factors: the incoming nucleophile would have to approach in the plane of the carbon–carbon double bond and burrow through part of the molecule to carry out a backside displacement.
Vinylic halides (R2C═CRX) and aryl halides are not shown on this reactivity list because they are unreactive toward SN2 displacement. This lack of reactivity is due to steric factors: the incoming nucleophile would have to approach in the plane of the carbon–carbon double bond and burrow through part of the molecule to carry out a backside displacement.
The Nucleophile
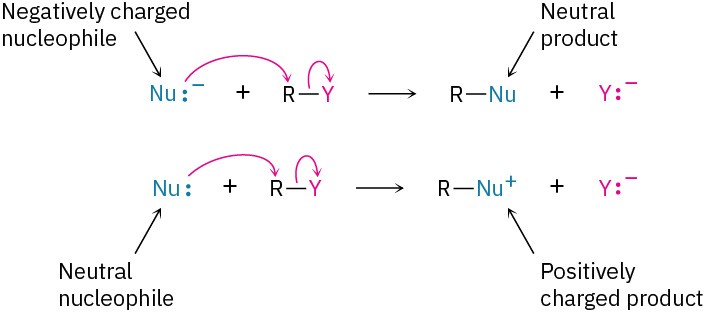
Another variable that has a major effect on the SN2 reaction is the nature of the nucleophile. Any species, either neutral or negatively charged, can act as a nucleophile as long as it has an unshared pair of electrons; that is, as long as it is a Lewis base. If the nucleophile is negatively charged, the product is neutral; if the nucleophile is neutral, the product is positively charged.
 A wide array of substances can be prepared using nucleophilic substitution reactions. In fact, we’ve already seen examples in previous chapters. For instance, the reaction of an acetylide anion with an alkyl halide, discussed in Section 5.14, is an SN2 reaction in which the acetylide nucleophile displaces a halide leaving group.
A wide array of substances can be prepared using nucleophilic substitution reactions. In fact, we’ve already seen examples in previous chapters. For instance, the reaction of an acetylide anion with an alkyl halide, discussed in Section 5.14, is an SN2 reaction in which the acetylide nucleophile displaces a halide leaving group.
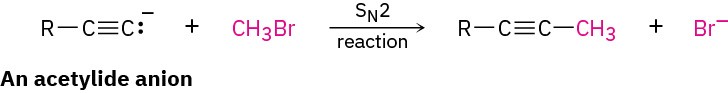 Table 7.1 lists some nucleophiles in the order of their reactivity, shows the products of their reactions with bromomethane, and gives the relative rates of their reactions. There are large differences in the rates at which various nucleophiles react.
Table 7.1 lists some nucleophiles in the order of their reactivity, shows the products of their reactions with bromomethane, and gives the relative rates of their reactions. There are large differences in the rates at which various nucleophiles react.
Table 7.1 Some SN2 Reactions with Bromomethane
|
Nu:– + CH3Br → CH3Nu + Br– |
||||
|
Nucleophile |
Product |
Relative rate of reaction
|
||
|
Formula |
Name |
Formula |
Name |
|
|
H2O |
Water |
CH3OH2+ |
Methylhydronium ion |
1 |
|
CH3CO2– |
Acetate |
CH3CO2CH3 |
Methyl acetate |
500 |
|
NH3 |
Ammonia |
CH3NH3+ |
Methylammonium ion |
700 |
|
Cl– |
Chloride |
CH3Cl |
Chloromethane |
1,000 |
|
HO– |
Hydroxide |
CH3OH |
Methanol |
10,000 |
|
Nucleophile |
Product |
Relative rate of reaction
|
||
|
Formula |
Name |
Formula |
Name |
|
|
CH3O– |
Methoxide |
CH3OCH3 |
Dimethyl ether |
25,000 |
|
I– |
Iodide |
CH3I |
Iodomethane |
100,000 |
|
–CN |
Cyanide |
CH3CN |
Acetonitrile |
125,000 |
|
HS– |
Hydrosulfide |
CH3SH |
Methanethiol |
125,000 |
What are the reasons for the reactivity differences observed in Table 7.1? Why do some reactants appear to be much more “nucleophilic” than others? The answers to these questions aren’t straightforward. Part of the problem is that the term nucleophilicity is imprecise. The term is usually taken to be a measure of the affinity of a nucleophile for a carbon atom in the SN2 reaction, but the reactivity of a given nucleophile can change from one reaction to the next. The exact nucleophilicity of a species in a given reaction depends on the substrate, the solvent, and even the reactant concentrations. Detailed explanations for the observed nucleophilicities aren’t always simple, but some trends can be detected from the data of Table 7.1.
Nucleophilicity roughly parallels basicity when comparing nucleophiles that have the same reacting atom. Thus, OH– is both more basic and more nucleophilic than acetate ion, CH3CO2–, which in turn is more basic and more nucleophilic than H2O. Since “nucleophilicity” is usually taken as the affinity of a Lewis base for a carbon atom in the SN2 reaction and “basicity” is the affinity of a base for a proton, it’s easy to see why there might be a correlation between the two kinds of behavior.
Nucleophilicity usually increases going down a column of the periodic table. Thus, HS– is more nucleophilic than HO–, and the halide reactivity order is I– > Br– > Cl–. Going down the periodic table, elements have their valence electrons in successively larger shells where they are successively farther from the nucleus, less tightly held, and consequently more reactive. This matter is complex, though, and the nucleophilicity order can change depending on the solvent.
Negatively charged nucleophiles are usually more reactive than neutral ones. As a result, SN2 reactions are often carried out under basic conditions rather than neutral or acidic conditions.
Problem 7.4
What product would you expect from SN2 reaction of 1-bromobutane with each of the following?
(a) NaI
(b) KOH
(c) H−C≡C-Li
(d) NH3
Problem 7.5
Which substance in each of the following pairs is more reactive as a nucleophile? Explain.
(a) (CH3)2N– or (CH3)2NH
(b) (CH3)3B or (CH3)3N
(c) H2O or H2S
The Leaving Group
Still another variable that can affect the SN2 reaction is the nature of the group displaced by the incoming nucleophile, the leaving group. Because the leaving group is expelled with a negative charge in most SN2 reactions, the best leaving groups are those that best stabilize the negative charge in the transition state. The greater the extent of charge stabilization by the leaving group, the lower the energy of the transition state and the more rapid the reaction. But as we saw in Section 1.18, the groups that best stabilize a negative charge are also the weakest bases. Thus, weak bases such as Cl–, Br–, and tosylate ion make good leaving groups, while strong bases such as OH– and NH2– make poor leaving groups.
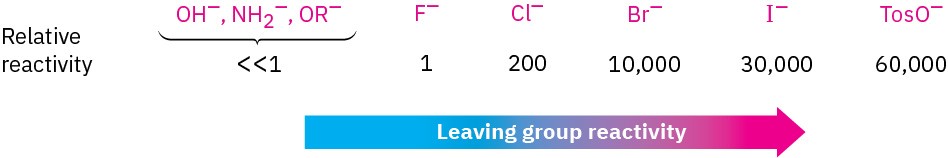 It’s just as important to know which are poor leaving groups as to know which are good, and the preceding data clearly indicate that F–, HO–, RO–, and H2N– are not displaced by nucleophiles. In other words, alkyl fluorides, alcohols, ethers, and amines do not typically undergo SN2 reactions. To carry out an SN2 reaction with an alcohol, it’s necessary to convert the –OH into a better leaving group. This, in fact, is just what happens when a primary or secondary alcohol is converted into either an alkyl chloride by reaction with SOCl2 or an alkyl bromide by reaction with PBr3.
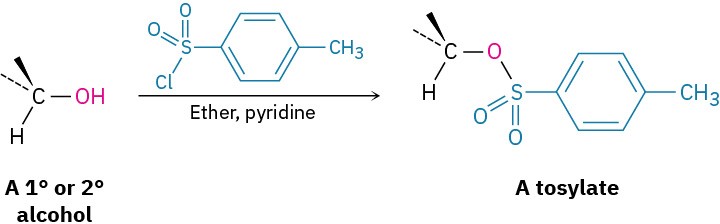
It’s just as important to know which are poor leaving groups as to know which are good, and the preceding data clearly indicate that F–, HO–, RO–, and H2N– are not displaced by nucleophiles. In other words, alkyl fluorides, alcohols, ethers, and amines do not typically undergo SN2 reactions. To carry out an SN2 reaction with an alcohol, it’s necessary to convert the –OH into a better leaving group. This, in fact, is just what happens when a primary or secondary alcohol is converted into either an alkyl chloride by reaction with SOCl2 or an alkyl bromide by reaction with PBr3.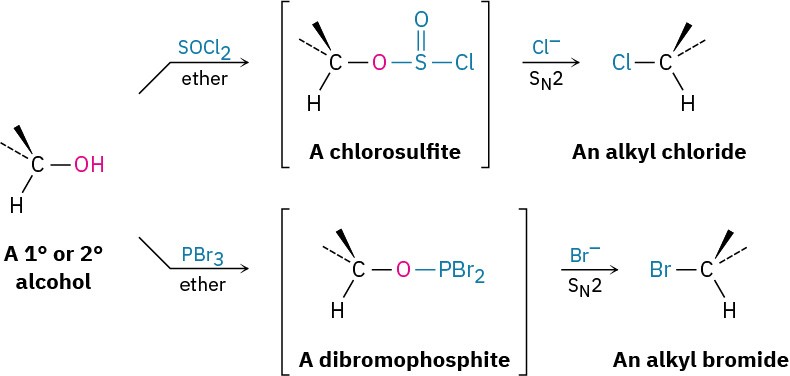 Alternatively, an alcohol can be made more reactive toward nucleophilic substitution by treating it with para-toluenesulfonyl chloride to form a tosylate. As noted previously, tosylates are even more reactive than halides in nucleophilic substitutions. Note that tosylate formation does not change the configuration of the oxygen-bearing carbon because the C–O bond is not broken.
Alternatively, an alcohol can be made more reactive toward nucleophilic substitution by treating it with para-toluenesulfonyl chloride to form a tosylate. As noted previously, tosylates are even more reactive than halides in nucleophilic substitutions. Note that tosylate formation does not change the configuration of the oxygen-bearing carbon because the C–O bond is not broken.
 Problem 7.6
Problem 7.6
Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)3CCl, (CH3)2CHCl
A Summary of SN2 Reaction Characteristics
The effects on SN2 reactions of the four variables—substrate structure, nucleophile, leaving group, and solvent—are summarized in the following statements:
|
Substrate |
Steric hindrance raises the energy of the SN2 transition state. As a result, SN2 reactions are best for methyl and primary substrates. Secondary substrates react slowly, and tertiary substrates do not react by an SN2 mechanism. |
|
Nucleophile |
Basic, negatively charged nucleophiles are less stable and have a higher ground-state energy than neutral ones, decreasing ∆G‡ and increasing the SN2 reaction rate. |
|
Leaving group |
Good leaving groups (more stable anions) lower the energy of the transition state, decreasing ∆G‡ and increasing the SN2 reaction rate. |

