1.16 Rules for Resonance Forms
When first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. The following rules should be helpful:
RULE 1
Individual resonance forms are imaginary, not real. The real structure is a composite, or resonance hybrid, of the different forms. Species such as the acetate ion and benzene are no different from any other. They have single, unchanging structures, and they don’t switch back and forth between resonance forms. The only difference between these and other substances is in the way they are represented in drawings.
RULE 2
Resonance forms differ only in the placement of their π or nonbonding electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. In the acetate ion, for instance, the carbon atom is sp2-hybridized and the oxygen atoms remain in exactly the same place in both resonance forms. Only the positions of the π electrons in the C=O bond and the lone-pair electrons on oxygen differ from one form to another. This movement of electrons from one resonance structure to another can be indicated with curved arrows. A curved arrow always indicates the movement of electrons, not the movement of atoms. An arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow.
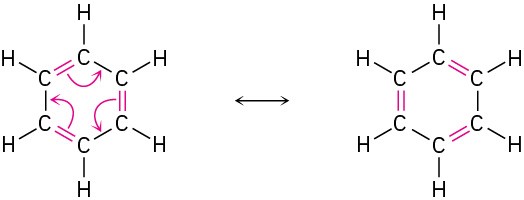
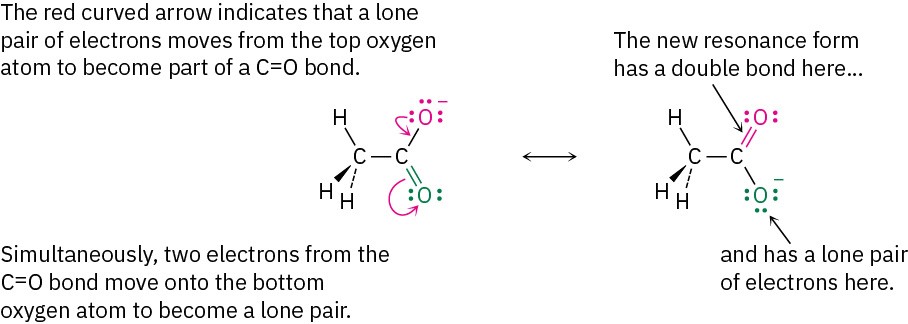 The situation with benzene is similar to that with acetate. The π electrons in the double bonds move, as shown with curved arrows, but the carbon and hydrogen atoms remain in place.
The situation with benzene is similar to that with acetate. The π electrons in the double bonds move, as shown with curved arrows, but the carbon and hydrogen atoms remain in place.
RULE 3
Different resonance forms of a substance don’t have to be equivalent. As an example, acetone, which contains a C=O bond, can be converted into its anion by reaction with a strong base. The resultant anion has two resonance forms. One form contains a carbon–oxygen double bond and has a negative charge on carbon; the other contains a carbon–carbon double bond and has a negative charge on oxygen. Even though the two resonance forms aren’t equivalent, both contribute to the overall resonance hybrid.
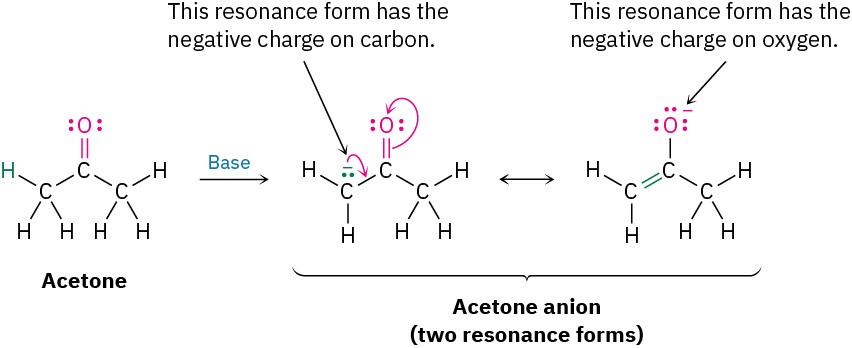 When two resonance forms are nonequivalent, the actual structure of the resonance hybrid resembles the more stable form. Thus, we might expect the true structure of the acetone anion to be more like that of the form that places the negative charge on the electronegative oxygen atom rather than on the carbon.
When two resonance forms are nonequivalent, the actual structure of the resonance hybrid resembles the more stable form. Thus, we might expect the true structure of the acetone anion to be more like that of the form that places the negative charge on the electronegative oxygen atom rather than on the carbon.
RULE 4
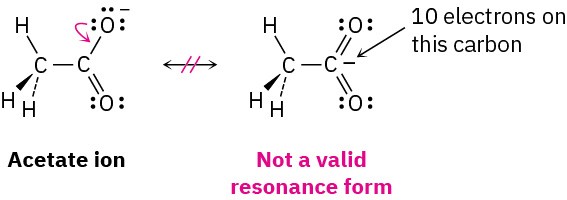
Resonance forms obey normal rules of valency. A resonance form is like any other structure: the octet rule still applies to second-row, main-group atoms. For example, one of the following structures for the acetate ion is not a valid resonance form because the carbon atom has five bonds and ten valence electrons:
RULE 5
The resonance hybrid is more stable than any individual resonance form. In other words, resonance leads to stability. Generally speaking, the larger the number of resonance forms a substance has, the more stable the substance is, because its electrons are spread out over a larger part of the molecule and are closer to more nuclei. For instance, a benzene ring is more stable because of resonance than might otherwise be expected.

