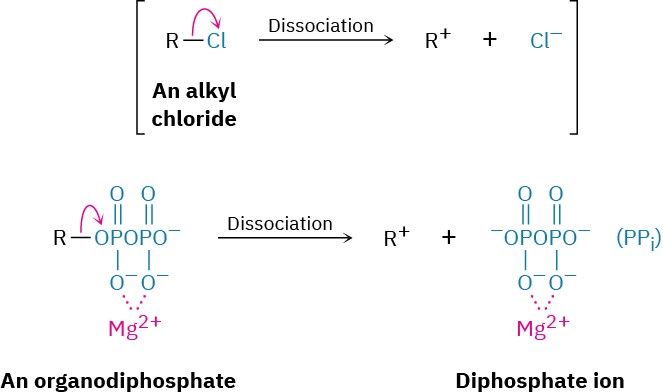
7.6 Biological Substitution Reactions
Both SN1 and SN2 reactions are common in biological chemistry, particularly in the pathways for biosynthesis of the many thousands of plant-derived substances called terpenoids, which we saw briefly in the Chapter 5 Chemistry Matters. Unlike what typically happens in the laboratory, however, the substrate in a biological substitution reaction is usually an organodiphosphate rather than an alkyl halide. Thus, the leaving group is the diphosphate ion, abbreviated PPi, rather than a halide ion. In fact, it’s useful to think of the diphosphate group as the “biological equivalent” of a halide.
The dissociation of an organodiphosphate in a biological reaction is typically assisted by complexation to a divalent metal cation such as Mg2+ to help neutralize charge and make the diphosphate a better leaving group.
 As an example, two SN1 reactions occur during the biosynthesis of geraniol, a fragrant alcohol found in roses and used in perfumery. Geraniol biosynthesis begins with dissociation of dimethylallyl diphosphate to give an allylic carbocation, which reacts with isopentenyl diphosphate (Figure 7.9). From the viewpoint of isopentenyl diphosphate, the reaction is an electrophilic alkene addition, but from the viewpoint of dimethylallyl diphosphate, the process is an SN1 reaction in which the carbocation intermediate reacts with a double bond as the nucleophile.
As an example, two SN1 reactions occur during the biosynthesis of geraniol, a fragrant alcohol found in roses and used in perfumery. Geraniol biosynthesis begins with dissociation of dimethylallyl diphosphate to give an allylic carbocation, which reacts with isopentenyl diphosphate (Figure 7.9). From the viewpoint of isopentenyl diphosphate, the reaction is an electrophilic alkene addition, but from the viewpoint of dimethylallyl diphosphate, the process is an SN1 reaction in which the carbocation intermediate reacts with a double bond as the nucleophile.
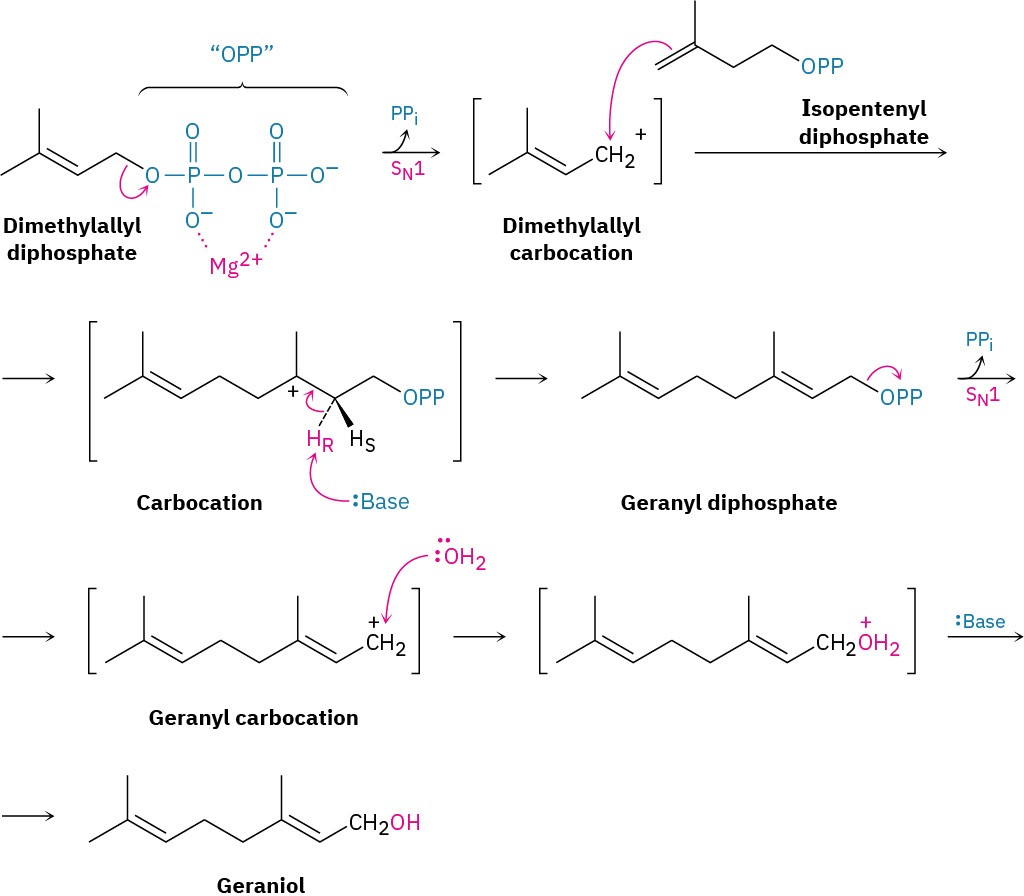 Figure 7.9 Biosynthesis of geraniol from dimethylallyl diphosphate. Two SN1 reactions occur, both with diphosphate ion as the leaving group.
Figure 7.9 Biosynthesis of geraniol from dimethylallyl diphosphate. Two SN1 reactions occur, both with diphosphate ion as the leaving group.
Following this initial SN1 reaction, loss of the pro-R hydrogen gives geranyl diphosphate, itself an allylic diphosphate that dissociates a second time. Reaction of the geranyl carbocation with water in a second SN1 reaction, followed by loss of a proton, then yields geraniol.
As another example, SN2 reactions are involved in almost all biological methylations, which transfer a –CH3 group from an electrophilic donor to a nucleophile. The donor is S-adenosylmethionine (abbreviated SAM), which contains a positively charged sulfur (a sulfonium ion, Section 3.10), and the leaving group is the neutral S-adenosylhomocysteine molecule. In the biosynthesis of epinephrine (adrenaline) from norepinephrine, for instance, the nucleophilic nitrogen atom of norepinephrine attacks the electrophilic methyl carbon atom of S-adenosylmethionine in an SN2 reaction, displacing S-adenosylhomocysteine (Figure 7.10). In effect, S-adenosylmethionine is simply a biological equivalent of CH3Cl.
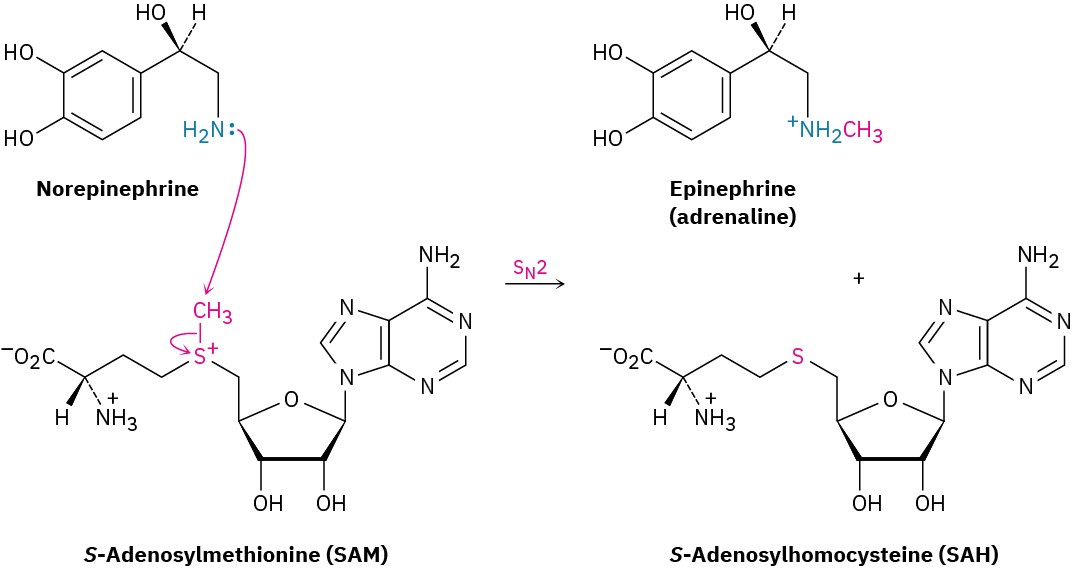
Figure 7.10 The biosynthesis of epinephrine from norepinephrine occurs by an SN2 reaction with S-adenosylmethionine.

