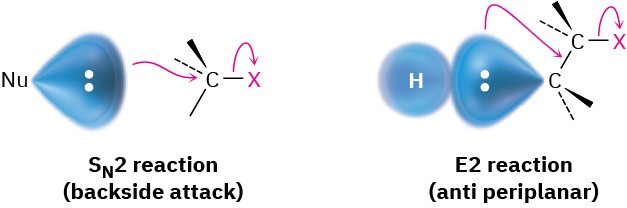
7.8 The E2 Reaction
The E2 reaction (for elimination, bimolecular) occurs when an alkyl halide is treated with a strong base, such as hydroxide ion or alkoxide ion (RO–). It is the most commonly occurring pathway for elimination and can be formulated as shown in Figure 7.11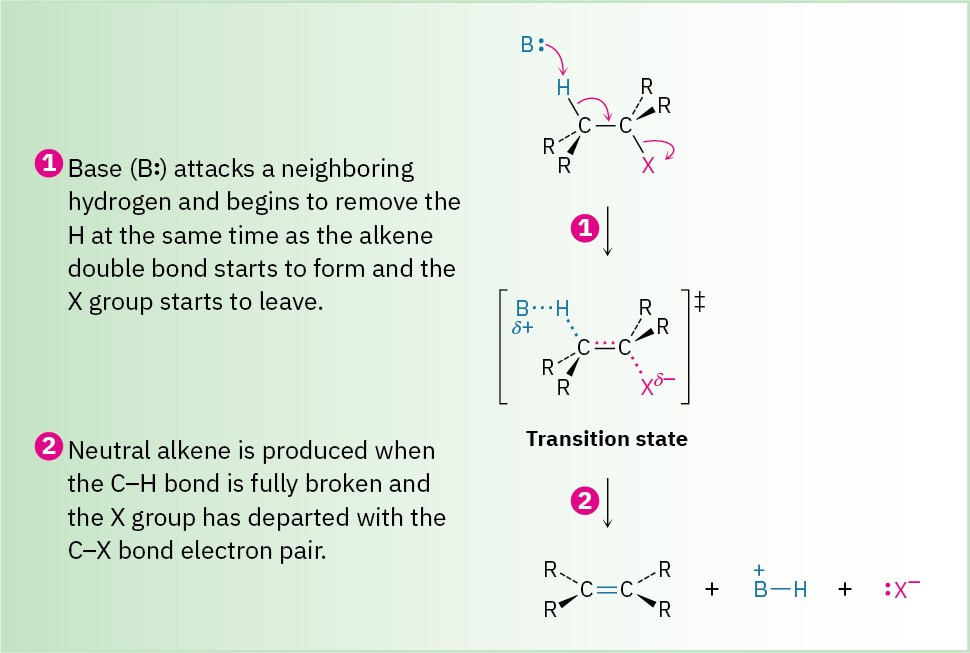
Figure 7.11 MECHANISM: Mechanism of the E2 reaction of an alkyl halide. The reaction takes place in a single step through a transition state in which the double bond begins to form at the same time the H and X groups are leaving.
Like the SN2 reaction, the E2 reaction takes place in one step without intermediates. As the base begins to abstract H+ from a carbon next to the leaving group, the C–H bond begins to break, a C═C bond begins to form, and the leaving group begins to depart, taking with it the electron pair from the C–X bond. Among the pieces of evidence supporting this mechanism is the fact that E2 reactions show second-order kinetics and follow the rate law: rate = k × [RX] × [Base]. That is, both the base and alkyl halide take part in the rate-limiting step.
As shown by a large number of experiments, E2 reactions occur with periplanar geometry, meaning that all four reacting atoms—the hydrogen, the two carbons, and the leaving group—lie in the same plane. Two such geometries are possible: syn periplanar geometry, in which the H and the X are on the same side of the molecule, and anti periplanar geometry, in which the H and the X are on opposite sides of the molecule. Of the two, anti periplanar geometry is energetically preferred because it allows the substituents on the two carbons to adopt a staggered relationship, whereas syn geometry requires that the substituents be eclipsed.
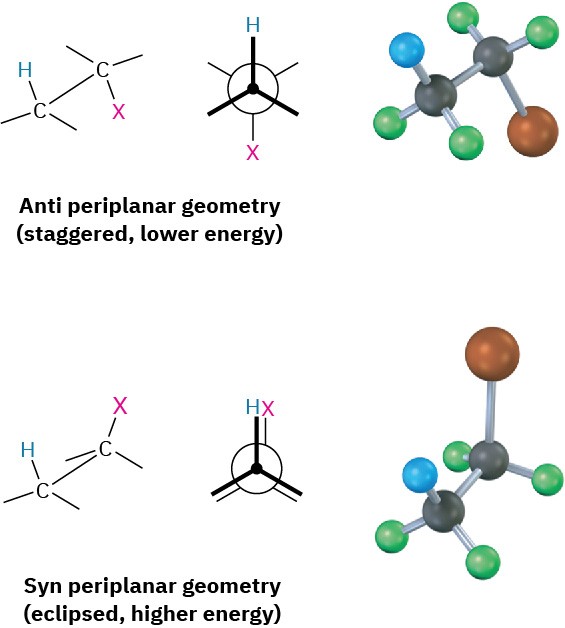 What’s so special about periplanar geometry? Because the sp3 σ orbitals in the reactant C–H and C–X bonds must overlap and become p π orbitals in the alkene product, there must also be some overlap in the transition state. This can occur most easily if all the orbitals are in the same plane to begin with—that is, if they’re periplanar (Figure 7.12).
What’s so special about periplanar geometry? Because the sp3 σ orbitals in the reactant C–H and C–X bonds must overlap and become p π orbitals in the alkene product, there must also be some overlap in the transition state. This can occur most easily if all the orbitals are in the same plane to begin with—that is, if they’re periplanar (Figure 7.12).
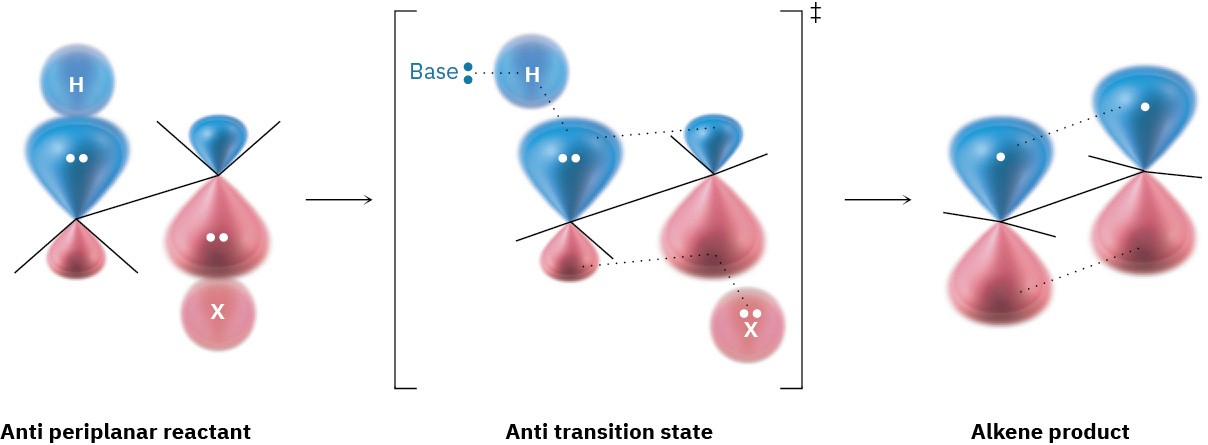
Figure 7.12 The transition state for the E2 reaction of an alkyl halide with base. Overlap of the developing p orbitals in the transition state requires periplanar geometry of the reactant.
You can think of E2 elimination reactions with periplanar geometry as being similar to SN2 reactions with 180° geometry. In an SN2 reaction, an electron pair from the incoming nucleophile pushes out the leaving group on the opposite side of the molecule. In an E2 reaction, an electron pair from a neighboring C–H bond also pushes out the leaving group on the opposite side of the molecule.