7.10 The E1 and E1cB Reactions
The E1 Reaction
Just as the E2 reaction is analogous to the SN2 reaction, the SN1 reaction has a close analog called the E1 reaction (for elimination, unimolecular). The E1 reaction can be formulated as shown in Figure 7.13, with the elimination of HCl from 2-chloro-2-methylpropane.
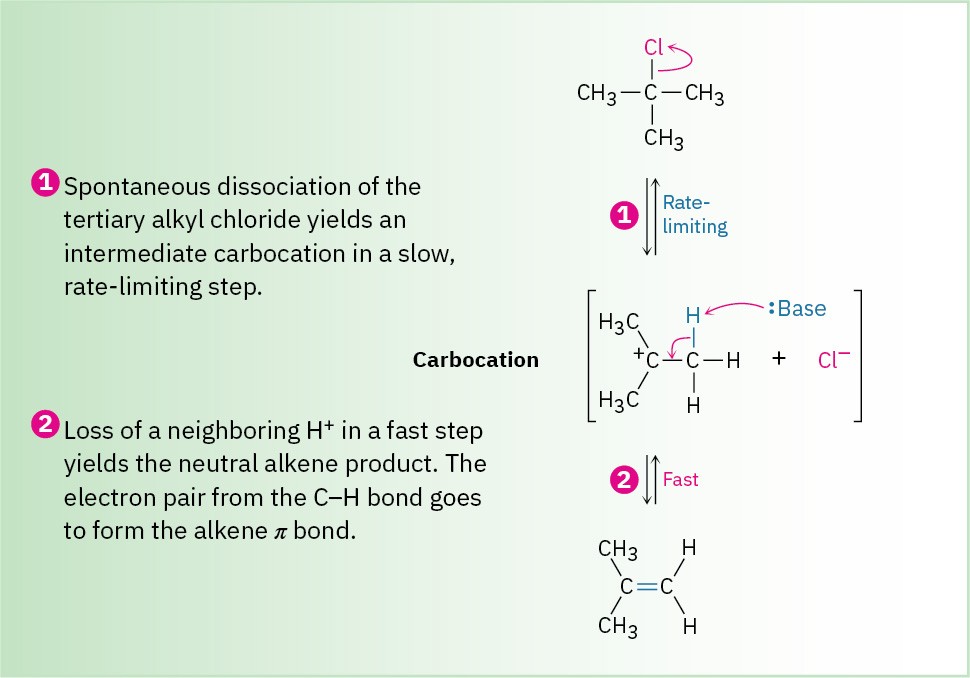
Figure 7.13 MECHANISM: Mechanism of the E1 reaction. Two steps are involved, the first of which is rate-limiting, and a carbocation intermediate is present.
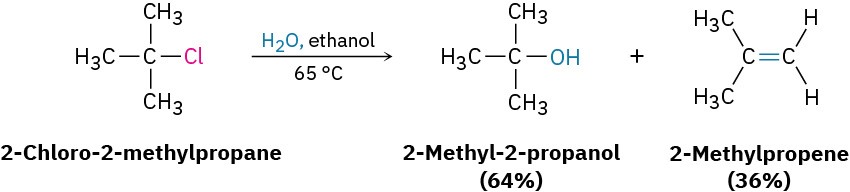
E1 eliminations begin with the same unimolecular dissociation to give a carbocation that we saw in the SN1 reaction, but the dissociation is followed by loss of H+ from the adjacent carbon rather than by substitution. In fact, the E1 and SN1 reactions normally occur together whenever an alkyl halide is treated in a protic solvent with a nonbasic nucleophile. Thus, the best E1 substrates are also the best SN1 substrates, and mixtures of substitution and elimination products are usually obtained. For example, when 2-chloro-2- methylpropane is warmed to 65 °C in 80% aqueous ethanol, a 64 : 36 mixture of 2-methyl- 2-propanol (SN1) and 2-methylpropene (E1) results.
 Much evidence has been obtained in support of the E1 mechanism. For example, E1 reactions show first-order kinetics, consistent with a rate-limiting, unimolecular dissociation process.
Much evidence has been obtained in support of the E1 mechanism. For example, E1 reactions show first-order kinetics, consistent with a rate-limiting, unimolecular dissociation process.
The E1cB Reaction
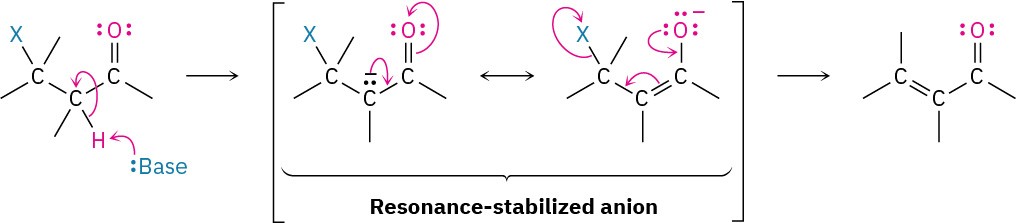
In contrast to the E1 reaction, which involves a carbocation intermediate, the E1cB reaction takes place through a carbanion intermediate. Base-induced abstraction of a proton in a slow, rate-limiting step gives an anion, which expels a leaving group on the adjacent carbon. The reaction is particularly common in substrates that have a poor leaving group, such as –OH, two carbons removed from a carbonyl group, as in HOC–CH–C═O. The poor leaving group disfavors the alternative E1 and E2 possibilities, and the carbonyl group makes the adjacent hydrogen unusually acidic by resonance stabilization of the anion intermediate.