5.8 Stability of Conjugated Dienes: Molecular Orbital Theory
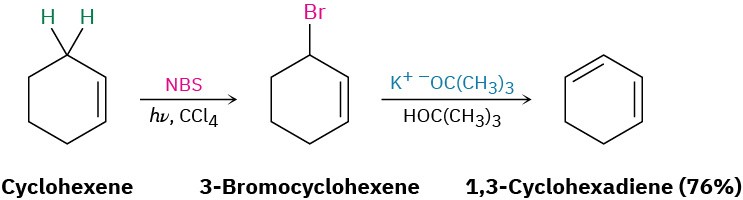
Conjugated dienes can be prepared by some of the methods previously discussed for preparing alkenes (Section 5.1–Section 5.2). The base-induced elimination of HX from an allylic halide is one such reaction.
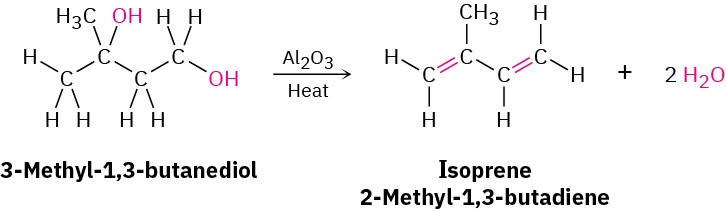
Simple conjugated dienes used in polymer synthesis include 1,3-butadiene, chloroprene (2-chloro-1,3-butadiene), and isoprene (2-methyl-1,3-butadiene). Isoprene has been prepared industrially by several methods, including the acid-catalyzed double dehydration of 3-methyl-1,3-butanediol.
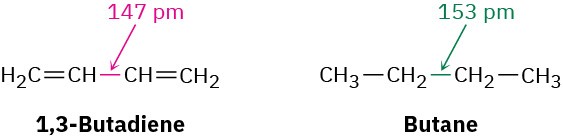
 One of the properties that distinguishes conjugated from nonconjugated dienes is that the central single bond is shorter than might be expected. The C2–C3 single bond in 1,3- butadiene, for instance, has a length of 147 pm, some 6 pm shorter than the C2–C3 bond in butane (153 pm).
One of the properties that distinguishes conjugated from nonconjugated dienes is that the central single bond is shorter than might be expected. The C2–C3 single bond in 1,3- butadiene, for instance, has a length of 147 pm, some 6 pm shorter than the C2–C3 bond in butane (153 pm).
 According to molecular orbital theory, the stability of conjugated dienes arises because of an interaction between the π orbitals of the two double bonds. To review briefly, when two p atomic orbitals combine to form a π bond, two π molecular orbitals (MOs) result. One is lower in energy than the starting p orbitals and is therefore bonding; the other is higher in energy, has a node between nuclei, and is antibonding. The two π electrons occupy the low-energy, bonding orbital, resulting in formation of a stable bond between atoms (Figure 5.9).
According to molecular orbital theory, the stability of conjugated dienes arises because of an interaction between the π orbitals of the two double bonds. To review briefly, when two p atomic orbitals combine to form a π bond, two π molecular orbitals (MOs) result. One is lower in energy than the starting p orbitals and is therefore bonding; the other is higher in energy, has a node between nuclei, and is antibonding. The two π electrons occupy the low-energy, bonding orbital, resulting in formation of a stable bond between atoms (Figure 5.9).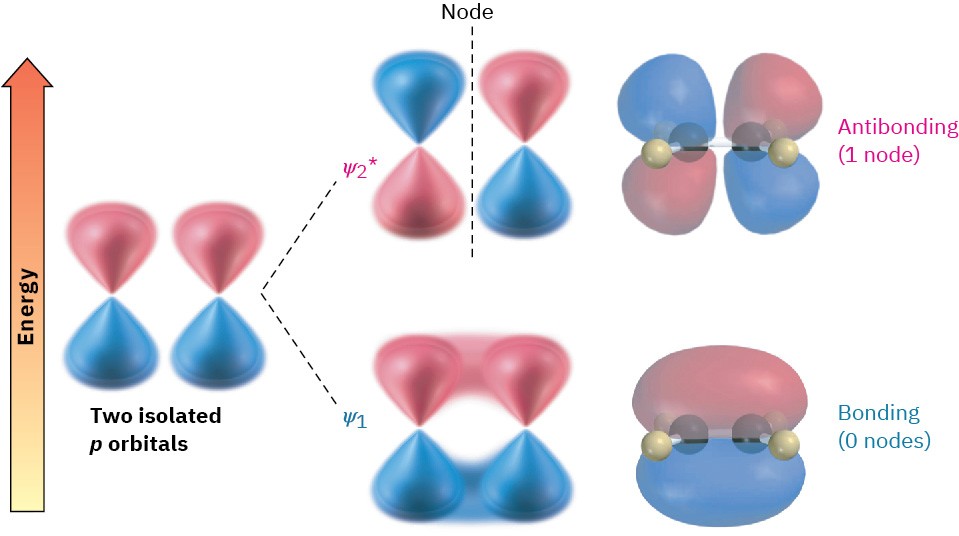 Figure 5.9 Two p orbitals combine to form two π molecular orbitals. Both electrons occupy the low-energy, bonding orbital, leading to a net lowering of energy and formation of a stable bond. The asterisk on ψ* indicates an antibonding orbital.
Figure 5.9 Two p orbitals combine to form two π molecular orbitals. Both electrons occupy the low-energy, bonding orbital, leading to a net lowering of energy and formation of a stable bond. The asterisk on ψ* indicates an antibonding orbital.
Now let’s combine four adjacent p atomic orbitals, as occurs in a conjugated diene. In so doing, we generate a set of four π molecular orbitals, two of which are bonding and two of which are antibonding (Figure 5.10). The four π electrons occupy the two bonding orbitals, leaving the antibonding orbitals vacant.
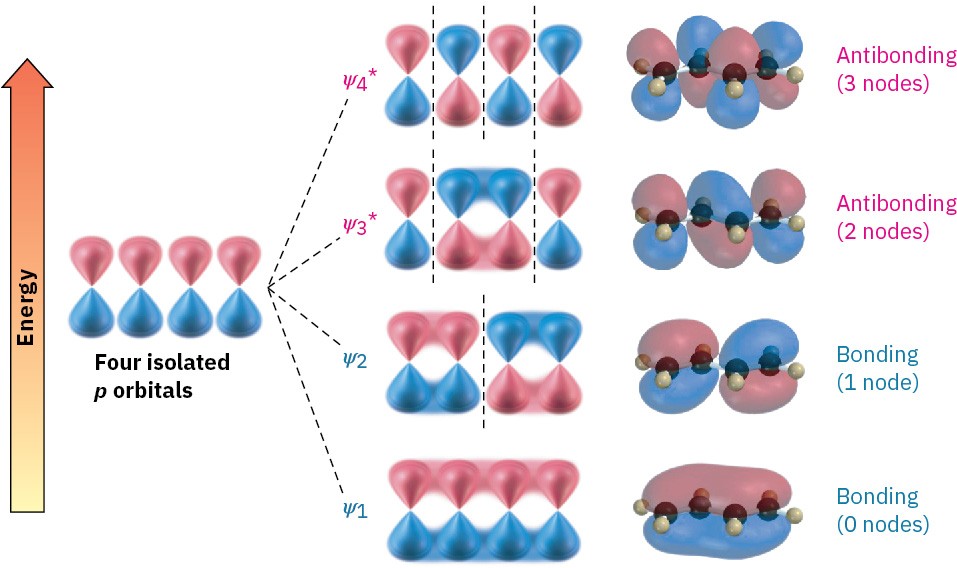 Figure 5.10 Four π molecular orbitals in 1,3-butadiene. Note that the number of nodes between nuclei increases as the energy level of the orbital increases.
Figure 5.10 Four π molecular orbitals in 1,3-butadiene. Note that the number of nodes between nuclei increases as the energy level of the orbital increases.
The lowest-energy π molecular orbital (denoted ψ1, Greek psi) has no nodes between the nuclei and is therefore bonding. The π MO of next-lowest energy, ψ2, has one node between nuclei and is also bonding. Above ψ1 and ψ2 in energy are the two antibonding π MOs, ψ3* and ψ4* (The asterisks indicate antibonding orbitals). Note that the number of nodes between nuclei increases as the energy level of the orbital increases. The ψ3* orbital has two nodes between nuclei, and ψ4*, the highest-energy MO, has three nodes between nuclei.
Comparing the π molecular orbitals of 1,3-butadiene (two conjugated double bonds) with those of 1,4-pentadiene (two isolated double bonds) shows why the conjugated diene is more stable. In a conjugated diene, the lowest-energy π MO (ψ1) has a favorable bonding interaction between C2 and C3 that is absent in a nonconjugated diene. As a result, there is a certain amount of double-bond character to the C2–C3 single bond, making that bond both stronger and shorter than a typical single bond. Electrostatic potential maps show clearly the additional electron density in the central single bond (Figure 5.11).
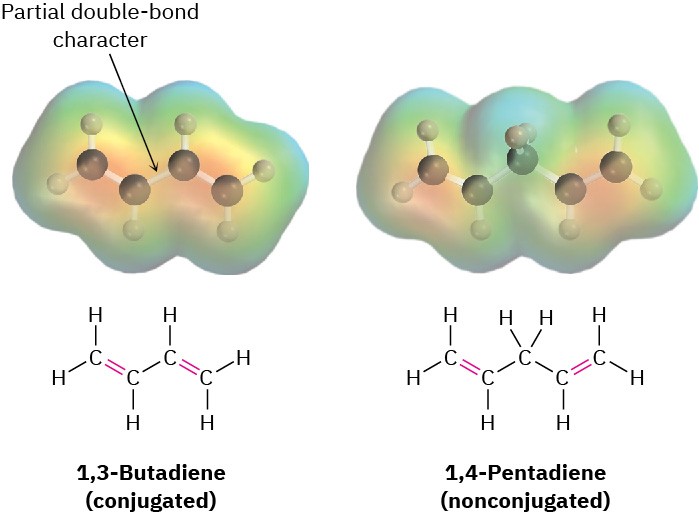 Figure 5.11 Electrostatic potential maps of 1,3-butadiene (conjugated) and 1,4- pentadiene (nonconjugated). Additional electron density is present in the central C−C bond of 1,3-butadiene, corresponding to partial double-bond character.
Figure 5.11 Electrostatic potential maps of 1,3-butadiene (conjugated) and 1,4- pentadiene (nonconjugated). Additional electron density is present in the central C−C bond of 1,3-butadiene, corresponding to partial double-bond character.
In describing 1,3-butadiene, we say that the π electrons are spread out, or delocalized, over the entire π framework, rather than localized between two specific nuclei. Delocalization allows the bonding electrons to be closer to more nuclei, thus leading to lower energy and greater stability.

