8.9 Other Aromatic Compounds
Aromatic compounds aren’t limited to benzene. Some aromatic compounds are heterocycles, a cyclic compound that contains atoms of more than one element in its ring, usually carbon along with nitrogen, oxygen, or sulfur. Pyridine and pyrimidine, for example, are six- membered heterocycles with carbon and nitrogen in their rings (Figure 8.17).
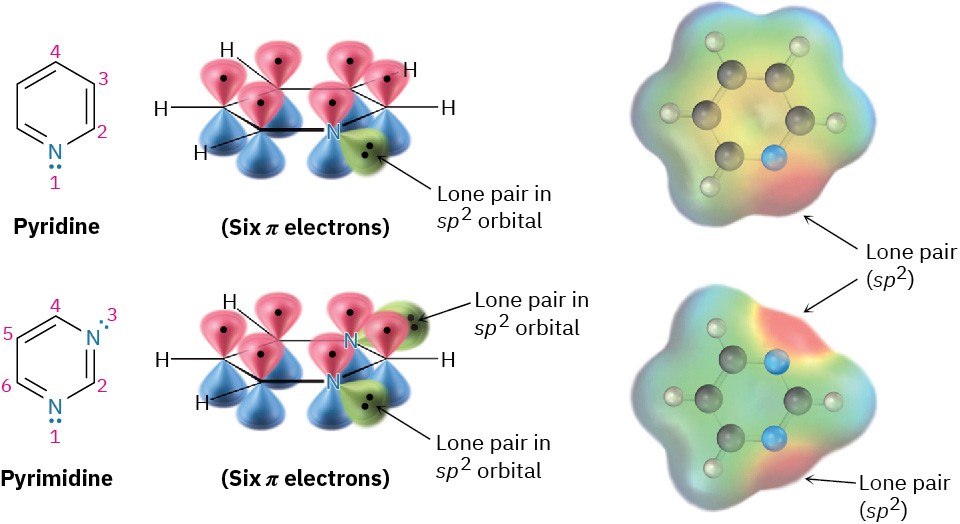 Figure 8.17 Pyridine and pyrimidine are nitrogen-containing aromatic heterocycles with π electron arrangements like that of benzene. Both have a lone pair of electrons on nitrogen in an sp2 orbital in the plane of the ring.
Figure 8.17 Pyridine and pyrimidine are nitrogen-containing aromatic heterocycles with π electron arrangements like that of benzene. Both have a lone pair of electrons on nitrogen in an sp2 orbital in the plane of the ring.
Pyridine is much like benzene in its π electron structure. Each of the five sp2-hybridized carbons has a p orbital perpendicular to the plane of the ring, and each p orbital contains one π electron. The nitrogen atom is also sp2-hybridized and has one electron in a p orbital, bringing the total to six π electrons. The nitrogen lone-pair electrons (red in an electrostatic potential map) are in a sp2 orbital in the plane of the ring and are not part of the aromatic π system. Pyrimidine, also shown in Figure 8.17), is a benzene analog that has two nitrogen atoms in a six-membered, unsaturated ring. Both nitrogens are sp2-hyridized, and each contributes one electron to the aromatic π system.
Pyrrole (spelled with two r’s and one l) and imidazole are five-membered heterocycles, yet both have six π electrons and are aromatic. In pyrrole, each of the four sp2-hybridized carbons contributes one π electron and the sp2-hybridized nitrogen atom contributes the two from its lone pair, which occupies a p orbital (Figure 8.18). Imidazole, also shown in (Figure 8.18, is an analog of pyrrole that has two nitrogen atoms in a five-membered, unsaturated ring. Both nitrogens are sp2-hybridized, but one is in a double bond and contributes only one electron to the aromatic π system whereas the other is not in a double bond and contributes two from its lone pair.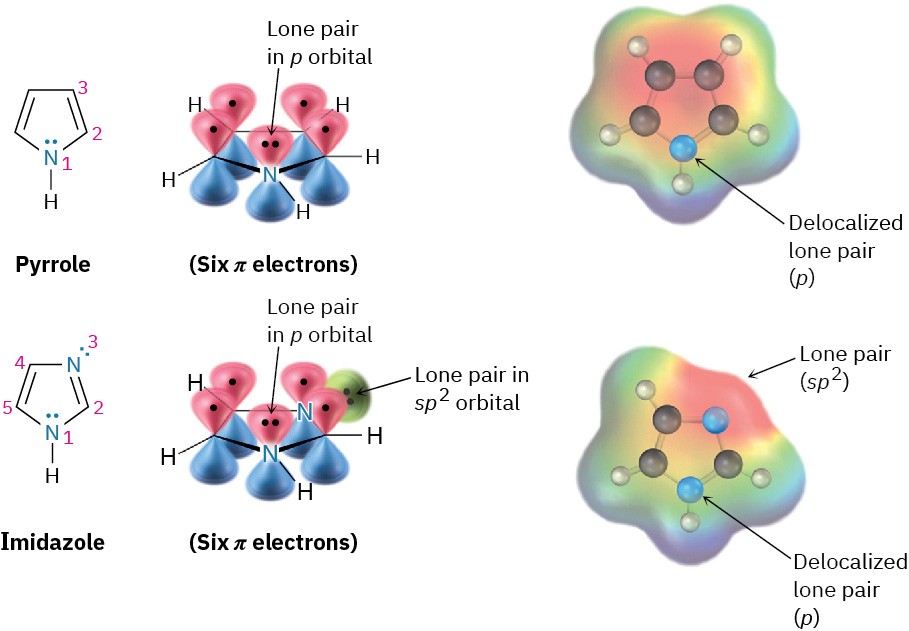 Figure 8.18 Pyrrole and imidazole are five-membered, nitrogen-containing heterocycles but have six-π-electron arrangements like that of the cyclopentadienyl anion. Both have a lone pair of electrons on nitrogen in a p orbital perpendicular to the ring.
Figure 8.18 Pyrrole and imidazole are five-membered, nitrogen-containing heterocycles but have six-π-electron arrangements like that of the cyclopentadienyl anion. Both have a lone pair of electrons on nitrogen in a p orbital perpendicular to the ring.
Compounds other than monocyclic compounds, like benzene, can be aromatic. Naphthalene, with two benzene-like rings fused together; anthracene, with three rings; benzo[a]pyrene, with five rings; and coronene, with six rings, are all well-known aromatic hydrocarbons. It is a polycyclic aromatic compounds Benzo[a]pyrene is particularly interesting because it is one of the cancer- causing substances found in tobacco smoke.
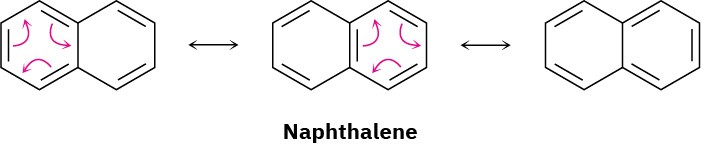
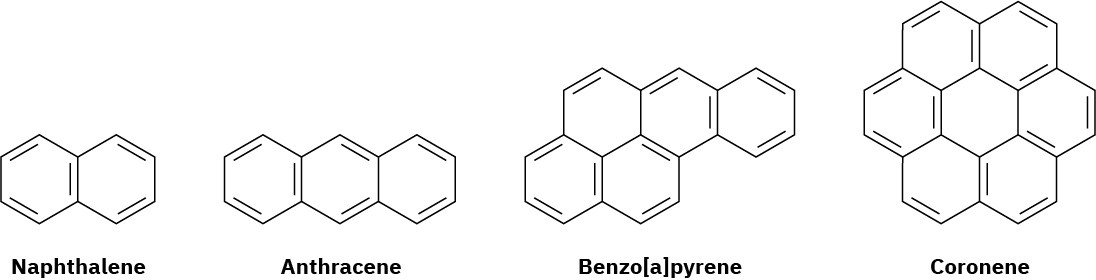 All polycyclic aromatic hydrocarbons can be represented by a number of different resonance forms. Naphthalene, for instance, has three.
All polycyclic aromatic hydrocarbons can be represented by a number of different resonance forms. Naphthalene, for instance, has three.
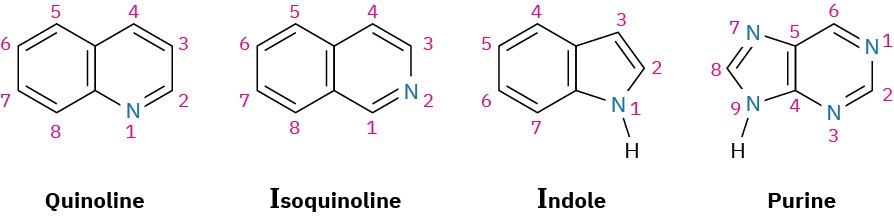
 Just as there are heterocyclic analogs of benzene, there are also many heterocyclic analogs of naphthalene. Among the most common are quinoline, isoquinoline, indole, and purine. Quinoline, isoquinoline, and purine all contain pyridine-like nitrogens that are part of a double bond and contribute one electron to the aromatic π system. Indole and purine both contain pyrrole-like nitrogens that contribute two π electrons.
Just as there are heterocyclic analogs of benzene, there are also many heterocyclic analogs of naphthalene. Among the most common are quinoline, isoquinoline, indole, and purine. Quinoline, isoquinoline, and purine all contain pyridine-like nitrogens that are part of a double bond and contribute one electron to the aromatic π system. Indole and purine both contain pyrrole-like nitrogens that contribute two π electrons.
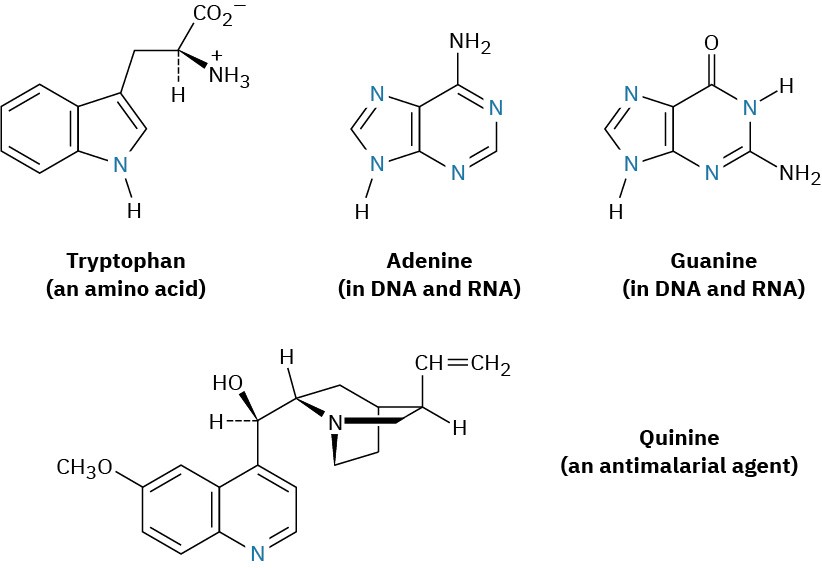
Among the many biological molecules that contain polycyclic aromatic rings, the amino acid tryptophan contains an indole ring and the antimalarial drug quinine contains a quinoline ring. Adenine and guanine, two of the five heterocyclic amine bases found in nucleic acids, have rings based on purine.