Chemistry Matters — Aspirin, NSAIDs, and COX-2 Inhibitors
Whatever the cause—whether tennis elbow, a sprained ankle, or a wrenched knee—pain and inflammation seem to go together. They are, however, different in their origin, and powerful drugs are available for treating each separately. Codeine, for example, is a powerful analgesic, or pain reliever, used in the management of debilitating pain, while cortisone and related steroids are potent anti-inflammatory agents, used for treating arthritis and other crippling inflammations. For minor pains and inflammation, both problems are often treated together by using a common over-the-counter medication called an NSAID, or nonsteroidal anti-inflammatory drug.
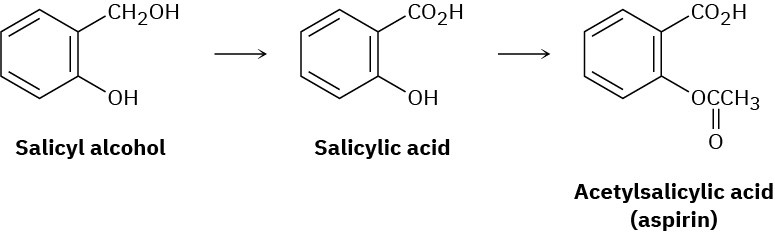
One of the most common NSAIDs is aspirin, or acetylsalicylic acid, whose use goes back to the late 1800s. It had been known from before the time of Hippocrates in 400 BC that fevers could be lowered by chewing the bark of willow trees. The active agent in willow bark was found in 1827 to be an aromatic compound called salicin, which could be converted by reaction with water into salicyl alcohol and then oxidized to give salicylic acid. Salicylic acid turned out to be even more effective than salicin for reducing fevers and to have analgesic and anti-inflammatory action as well. Unfortunately, it also turned out to be too corrosive to the walls of the stomach for everyday use. Conversion of the phenol – OH group into an acetate ester, however, yielded acetylsalicylic acid, which proved just as potent as salicylic acid but less corrosive to the stomach.

Figure 8.19 Many athletes rely on NSAIDs to help with pain and soreness. (credit: “Dillon Gee delivers a pitch” by Arturo Pardavila III/Wikimedia Commons, CC BY 2.0)
 Although extraordinary in its effect, aspirin is also more dangerous than commonly believed. 15 g of aspirin would be fatal to a child of 9–10 years, and 10 g would be lethal for a 6-year-old, and aspirin can cause stomach bleeding and allergic reactions in long-term users. Even more serious is a condition called Reye’s syndrome, a potentially fatal reaction to aspirin sometimes seen in children recovering from the flu. As a result of these problems, numerous other NSAIDs have been developed in the last several decades, most notably ibuprofen and naproxen.
Although extraordinary in its effect, aspirin is also more dangerous than commonly believed. 15 g of aspirin would be fatal to a child of 9–10 years, and 10 g would be lethal for a 6-year-old, and aspirin can cause stomach bleeding and allergic reactions in long-term users. Even more serious is a condition called Reye’s syndrome, a potentially fatal reaction to aspirin sometimes seen in children recovering from the flu. As a result of these problems, numerous other NSAIDs have been developed in the last several decades, most notably ibuprofen and naproxen.
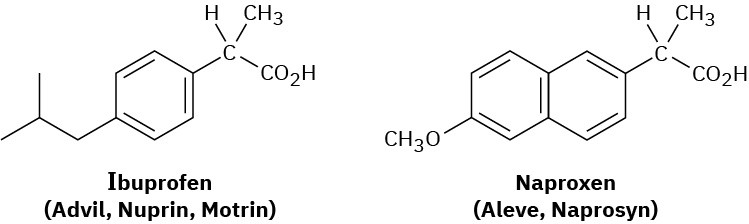
Like aspirin, both ibuprofen and naproxen are relatively simple aromatic compounds containing a side-chain carboxylic acid group. Ibuprofen, sold under the brand names Advil, Nuprin, Motrin, and others, has roughly the same potency as aspirin but is less prone to causing an upset stomach. Naproxen, sold under the names Aleve and Naprosyn, also has about the same potency as aspirin but remains active in the body six times longer.
Aspirin and other NSAIDs function by blocking the cyclooxygenase (COX) enzymes that carry out the body’s synthesis of prostaglandins. There are two forms of the enzyme: COX-1, which carries out the normal physiological production of prostaglandins, and COX-2, which mediates the body’s response to arthritis and other inflammatory conditions. Unfortunately, both COX-1 and COX-2 enzymes are blocked by aspirin, ibuprofen, and other NSAIDs, thereby shutting down not only the response to inflammation but also various protective functions, including the control mechanism for production of acid in the stomach.
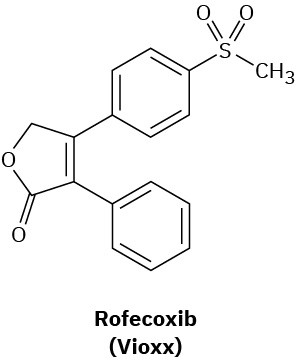
Medicinal chemists have therefore devised a number of drugs that act as selective inhibitors of only the COX-2 enzyme. Inflammation is thereby controlled without blocking protective functions. Originally heralded as a breakthrough in arthritis treatment, the first generation of COX-2 inhibitors, including rofecoxib, (marketed as Vioxx), celecoxib, (marketed as Celebrex), and Valdecoxib (marketed as Bextra), turned out to cause potentially serious heart problems, particularly in elderly or compromised patients. As a result, all three Cox-2 inhibitors were removed from the U.S. and other markets. Further studies, however, concluded that in proper doses the Cox-2 inhibitors were little different from other NSAIDs, and Vioxx returned to the market in 2017 for topical treatment of osteoarthritis.