Summary
Aromatic rings are a common part of many biological structures and are particularly important in nucleic acid chemistry and in the chemistry of several amino acids. In this chapter, we’ve seen how and why aromatic compounds are different from such apparently related compounds as cycloalkenes.
The word aromatic is used for historical reasons to refer to the class of compounds related structurally to benzene. Aromatic compounds are systematically named according to IUPAC rules, but many common names are also used. Disubstituted benzenes are referred to as ortho (1,2 disubstituted), meta (1,3 disubstituted), or para (1,4 disubstituted) derivatives. The C6H5– unit itself is referred to as a phenyl group, and the C6H5CH2– unit is a benzyl group.
Benzene is described by valence-bond theory as a resonance hybrid of two equivalent structures and is described by molecular orbital theory as a planar, cyclic, conjugated molecule with six π electrons.
Other substances besides benzene-like compounds are also aromatic. Pyridine and pyrimidine are six-membered, nitrogen-containing, aromatic heterocycles. Pyrrole and imidazole are five-membered, nitrogen-containing heterocycles. Naphthalene, quinoline, indole, and many others are polycyclic aromatic compounds.
An electrophilic aromatic substitution reaction takes place in two steps—initial reaction of an electrophile, E+, with the aromatic ring, followed by loss of H+ from the resonance-stabilized carbocation intermediate to regenerate the aromatic ring.
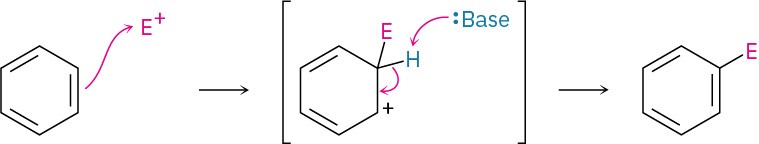
Many variations of the reaction can be carried out, including halogenation, nitration, and sulfonation. Friedel–Crafts alkylation and acylation reactions, which involve reaction of an aromatic ring with carbocation electrophiles, are particularly useful. They are limited, however, by the fact that the aromatic ring must be at least as reactive as a halobenzene.
Substituents on the benzene ring affect both the reactivity of the ring toward further substitution and the orientation of that substitution. Groups can be classified as ortho- and para-directing activators, ortho- and para-directing deactivators, or meta-directing deactivators. Substituents influence aromatic rings by a combination of resonance and inductive effects. Resonance effects are transmitted through π bonds; inductive effects are transmitted through σ bonds.

