8.6 Substituent Effects in Electrophilic Substitutions
Only one product can form when an electrophilic substitution occurs on benzene, but what would happen if we were to carry out a reaction on an aromatic ring that already has a substituent? The initial presence of a substituent on the ring has two effects.
- Substituents affect the reactivity of the aromatic ring. Some substituents activate the ring, making it more reactive than benzene, and some deactivate the ring, making it less reactive than benzene. In aromatic nitration, for instance, an –OH substituent makes the ring 1000 times more reactive than benzene, while an –NO2 substituent makes the ring more than 10 million times less reactive.
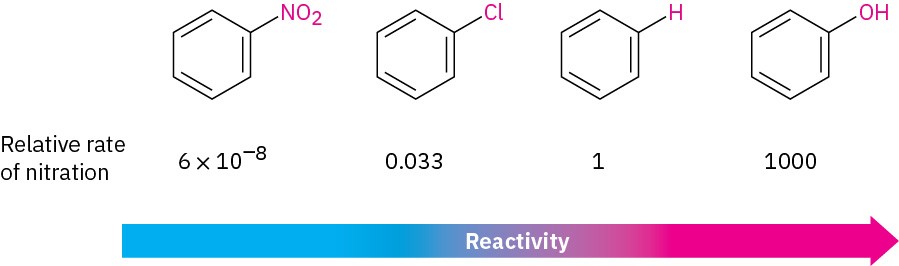
- Substituents affect the orientation of the reaction. The three possible disubstituted products—ortho, meta, and para—are usually not formed in equal amounts. Instead, the nature of the substituent initially present on the benzene ring determines the position of the second substitution. An –OH group directs substitution toward the ortho and para positions, for instance, while a carbonyl group such as –CHO directs substitution primarily toward the meta position.
Table 8.2 lists experimental results for the nitration of some substituted benzenes.
Table 8.2 Orientation of Nitration in Substituted Benzenes
|
|
|||
|
|
Product (%) |
||
|
|
Ortho |
Meta |
Para |
|
Meta-directing deactivators |
|||
| –+N(CH3)3 |
2 |
87 |
11 |
|
–NO2 |
7 |
91 |
2 |
|
–CO2H |
22 |
76 |
2 |
|
–CN |
17 |
81 |
2 |
|
–CO2CH3 |
28 |
66 |
6 |
|
–COCH3 |
26 |
72 |
2 |
|
–CHO |
19 |
72 |
9 |
|
Ortho- and para-directing deactivators |
|||
|
–F |
13 |
1 |
86 |
|
–Cl |
35 |
1 |
64 |
|
–Br |
43 |
1 |
56 |
|
–I |
45 |
1 |
54 |
|
Ortho- and para-directing activators |
|||
|
–CH3 |
63 |
3 |
34 |
|
–OH |
50 |
0 |
50 |
|
–NHCOCH3 |
19 |
2 |
79 |
Substituents can be classified into three groups, as shown in Figure 8.11: ortho- and para- directing activators, ortho- and para-directing deactivators, and meta-directing deactivators. There are no meta-directing activators. Notice how the directing effect of a group correlates with its reactivity. All meta-directing groups are strongly deactivating, and most ortho- and para-directing groups are activating. The halogens are unique in being ortho- and para-directing but weakly deactivating.
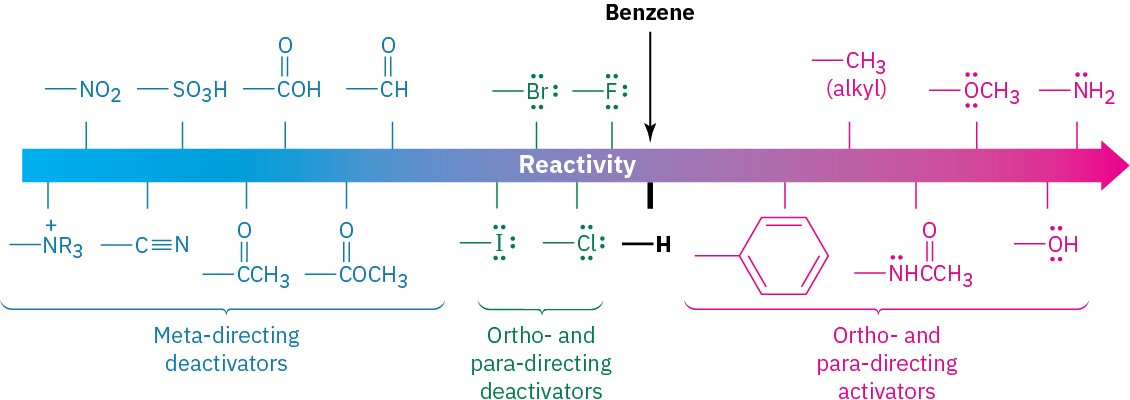 Figure 8.11 Classification of substituent effects in electrophilic aromatic substitution. All activating groups are ortho- and para-directing, and all deactivating groups other than halogen are meta-directing. Halogens are unique in being deactivating but ortho- and para-directing.
Figure 8.11 Classification of substituent effects in electrophilic aromatic substitution. All activating groups are ortho- and para-directing, and all deactivating groups other than halogen are meta-directing. Halogens are unique in being deactivating but ortho- and para-directing.
Worked Example 8.1: Predicting the Product of an Electrophilic Aromatic Substitution Reaction
Predict the major product of the sulfonation of toluene.
Strategy
Identify the substituent present on the ring, and decide whether it is ortho- and para- directing or meta-directing. According to Figure 8.11, an alkyl substituent is ortho- and para-directing, so sulfonation of toluene will primarily give a mixture of o-toluenesulfonic acid and p-toluenesulfonic acid.
Solution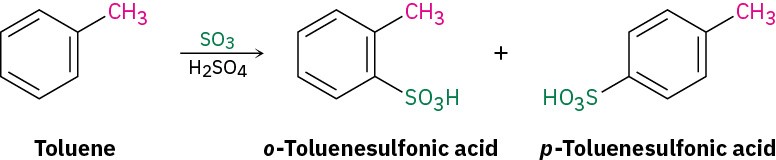
Problem 8.8
Rank the compounds in each of the following groups in order of their reactivity to electrophilic substitution:
(a) Nitrobenzene, phenol, toluene, benzene
(b) Phenol, benzene, chlorobenzene, benzoic acid
(c) Benzene, bromobenzene, benzaldehyde, aniline
Problem 8.9
Predict the major products of the following reactions:
(a) Nitration of bromobenzene
(b) Bromination of nitrobenzene
(c) Chlorination of phenol
(d) Bromination of aniline


