9.11 Preparing Ethers
Diethyl ether and other simple symmetrical ethers are prepared industrially by the sulfuric-acid-catalyzed reaction of alcohols. The reaction occurs by SN2 displacement of water from a protonated ethanol molecule by the oxygen atom of a second ethanol.
Unfortunately, this method is limited to use with primary alcohols because secondary and tertiary alcohols dehydrate by an E1 mechanism to yield alkenes (Section 9.7).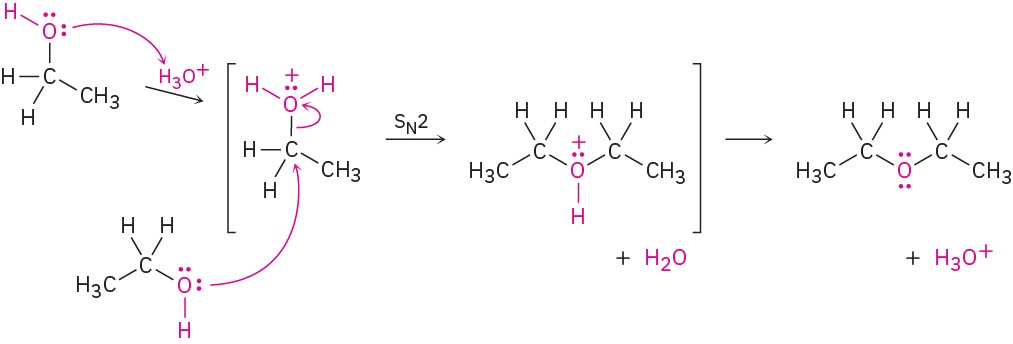
The Williamson Ether Synthesis
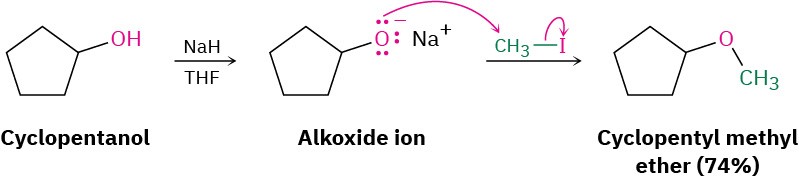
The most generally useful method of preparing ethers is the Williamson ether synthesis, in which an alkoxide ion reacts with a primary alkyl halide in an SN2 reaction. As we saw in Section 9.2, the alkoxide ion is normally prepared by reaction of an alcohol with a strong base such as sodium hydride, NaH.
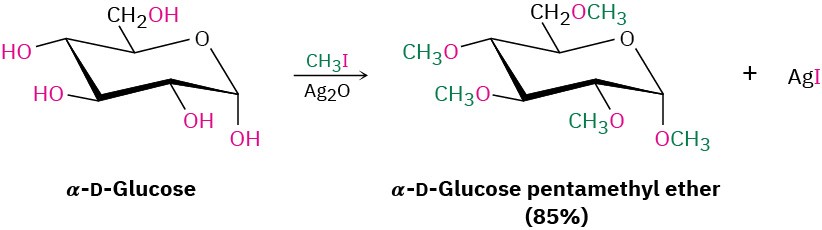
A useful variation of the Williamson synthesis involves silver oxide, Ag2O, as a mild base rather than NaH. Under these conditions, the free alcohol reacts directly with the alkyl halide, so there is no need to preform the metal alkoxide intermediate. Sugars react particularly well; glucose, for example, reacts with excess iodomethane in the presence of Ag2O to generate a pentaether in 85% yield.
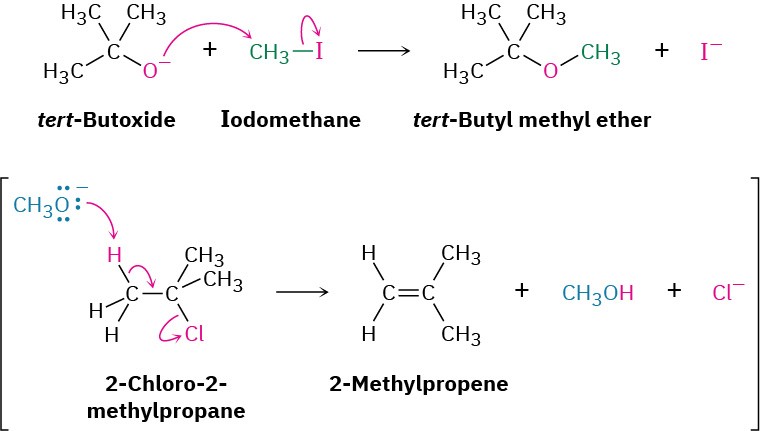
 Because the Williamson synthesis is an SN2 reaction, it is subject to all the usual constraints, as discussed in Section 7.3. Primary halides work best because competitive E2 elimination can occur with more hindered substrates. Unsymmetrical ethers should therefore be synthesized by reaction between the more hindered alkoxide partner and less hindered halide partner rather than vice versa. For example, tert-butyl methyl ether, a substance used in the 1990s as an octane booster in gasoline, is best prepared by reaction of tert-butoxide ion with iodomethane rather than by reaction of methoxide ion with 2-chloro-2-methylpropane.
Because the Williamson synthesis is an SN2 reaction, it is subject to all the usual constraints, as discussed in Section 7.3. Primary halides work best because competitive E2 elimination can occur with more hindered substrates. Unsymmetrical ethers should therefore be synthesized by reaction between the more hindered alkoxide partner and less hindered halide partner rather than vice versa. For example, tert-butyl methyl ether, a substance used in the 1990s as an octane booster in gasoline, is best prepared by reaction of tert-butoxide ion with iodomethane rather than by reaction of methoxide ion with 2-chloro-2-methylpropane.
 Problem 9.14
Problem 9.14
Why do you suppose only symmetrical ethers are prepared by the sulfuric-acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal reactivity?
Problem 9.15
How would you prepare the following ethers using a Williamson synthesis?
(a) Methyl propyl ether
(b) Anisole (methyl phenyl ether)
(c) Benzyl isopropyl ether
(d) Ethyl 2,2-dimethylpropyl ether
Problem 9.16
Rank the following halides in order of their reactivity in the Williamson synthesis:
(a) Bromoethane, 2-bromopropane, bromobenzene
(b) Chloroethane, bromoethane, 1-Iodopropene

