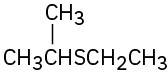9.15 Thiols and Sulfides
Thiols
Thiols, also called mercaptans, are sulfur analogs of alcohols. They are named by the same system used for alcohols, with the suffix –thiol used in place of –ol. The –SH group itself is referred to as a mercapto group. Like alcohols, thiols are weakly acidic; the pKa of CH3SH, for instance, is 10.3. Unlike alcohols, however, thiols don’t typically form hydrogen bonds because the sulfur atom is not sufficiently electronegative.
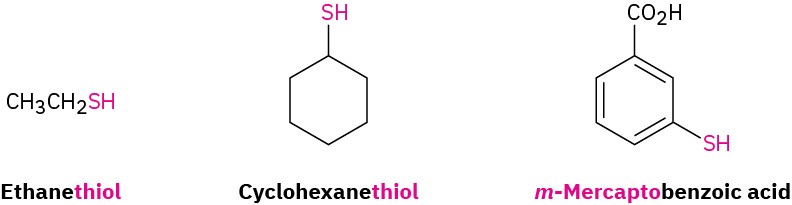 The most striking characteristic of thiols is their appalling odor. Skunk scent, for instance, is caused primarily by the simple thiols 3-methyl-1-butanethiol and 2-butene-1-thiol.
The most striking characteristic of thiols is their appalling odor. Skunk scent, for instance, is caused primarily by the simple thiols 3-methyl-1-butanethiol and 2-butene-1-thiol.
Volatile thiols such as ethanethiol are also added to natural gas and liquefied propane to serve as an easily detectable warning in case of leaks.
Thiols are usually prepared from alkyl halides by SN2 displacement with a sulfur nucleophile such as hydrosulfide anion, –SH.
 The reaction often works poorly unless an excess of the nucleophile is used because the product thiol can undergo a second SN2 reaction with alkyl halide to give a sulfide (R–S–Rʹ) as by-product.
The reaction often works poorly unless an excess of the nucleophile is used because the product thiol can undergo a second SN2 reaction with alkyl halide to give a sulfide (R–S–Rʹ) as by-product.
Sulfides
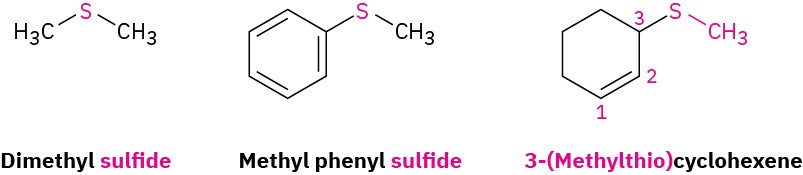
Sulfides are the sulfur analogs of ethers just as thiols are the sulfur analogs of alcohols. Sulfides are named by following the same rules used for ethers, with sulfide used in place of ether for simple compounds and alkylthio used in place of alkoxy for more complex substances.
 Treatment of a thiol with a base, such as NaH, gives the corresponding thiolate ion (RS–), which undergoes reaction with a primary or secondary alkyl halide to give a sulfide. The reaction occurs by an SN2 mechanism, analogous to the Williamson synthesis of ethers (Section 9.11).
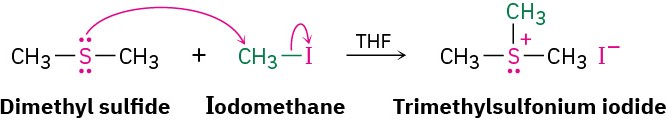
Treatment of a thiol with a base, such as NaH, gives the corresponding thiolate ion (RS–), which undergoes reaction with a primary or secondary alkyl halide to give a sulfide. The reaction occurs by an SN2 mechanism, analogous to the Williamson synthesis of ethers (Section 9.11).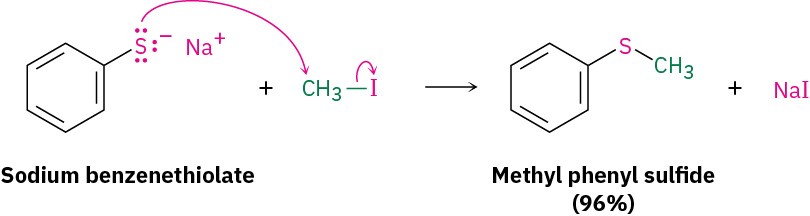 Despite their close structural similarity, sulfides and ethers differ substantially in their chemistry. Because the valence electrons on sulfur are farther from the nucleus and are less tightly held than those on oxygen (3p electrons versus 2p electrons), sulfur compounds are more nucleophilic than their oxygen analogs. Unlike dialkyl ethers, dialkyl sulfides react rapidly with primary alkyl halides by an SN2 mechanism to give sulfonium ions (R3S+).
Despite their close structural similarity, sulfides and ethers differ substantially in their chemistry. Because the valence electrons on sulfur are farther from the nucleus and are less tightly held than those on oxygen (3p electrons versus 2p electrons), sulfur compounds are more nucleophilic than their oxygen analogs. Unlike dialkyl ethers, dialkyl sulfides react rapidly with primary alkyl halides by an SN2 mechanism to give sulfonium ions (R3S+).
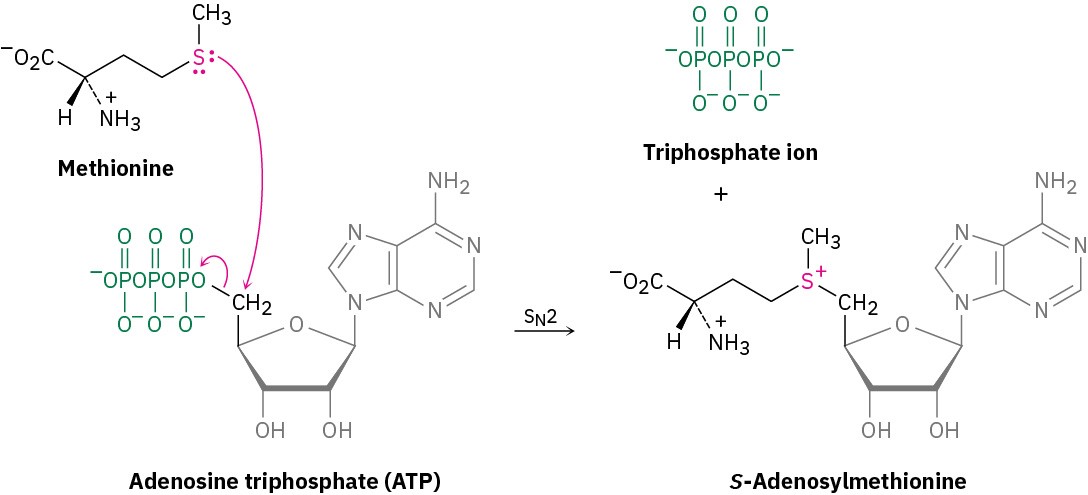
 The most common example of this process in living organisms is the reaction of the amino acid methionine with adenosine triphosphate (ATP) to give S-adenosylmethionine. The reaction is somewhat unusual in that the biological leaving group in this SN2 process is the triphosphate ion rather than the more frequently seen diphosphate ion (Section 7.6).
The most common example of this process in living organisms is the reaction of the amino acid methionine with adenosine triphosphate (ATP) to give S-adenosylmethionine. The reaction is somewhat unusual in that the biological leaving group in this SN2 process is the triphosphate ion rather than the more frequently seen diphosphate ion (Section 7.6).
 Sulfonium ions are themselves useful alkylating agents because a nucleophile can attack one of the groups bonded to the positively charged sulfur, displacing a neutral sulfide as leaving group. We saw an example of this in Section 7.6 (Figure 7.10), in which S-adenosylmethionine transferred a methyl group to norepinephrine to give adrenaline.
Sulfonium ions are themselves useful alkylating agents because a nucleophile can attack one of the groups bonded to the positively charged sulfur, displacing a neutral sulfide as leaving group. We saw an example of this in Section 7.6 (Figure 7.10), in which S-adenosylmethionine transferred a methyl group to norepinephrine to give adrenaline.
Problem 9.22
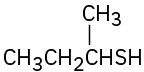
Name the following compounds:
(a)
(b)
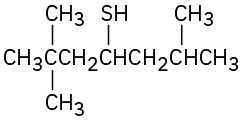
(c)
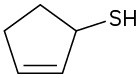
(d)