Chemistry Matters — Enantioselective Synthesis
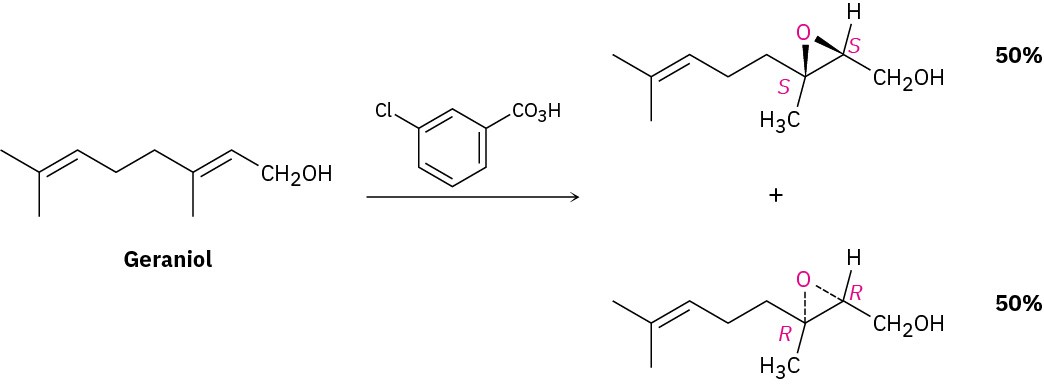
Whenever a chiral product is formed by reaction between achiral reagents, the product is racemic; that is, both enantiomers of the product are formed in equal amounts. The epoxidation reaction of geraniol with m-chloroperoxybenzoic acid, for instance, gives a racemic mixture of (2S,3S) and (2R,3R) epoxides.
 Unfortunately, it’s usually the case that only one enantiomer of a given drug or other important substance has the desired biological properties. The other enantiomer might be inactive or even dangerous. Thus, much work is currently being done on developing enantioselective methods of synthesis, which yield only one of the two possible enantiomers. So important has enantioselective synthesis become that the 2001 Nobel Prize in Chemistry was awarded to three pioneers in the field: William S. Knowles of the Monsanto Co., K. Barry Sharpless of the Scripps Research Institute, and Ryoji Noyori of Nagoya University.
Unfortunately, it’s usually the case that only one enantiomer of a given drug or other important substance has the desired biological properties. The other enantiomer might be inactive or even dangerous. Thus, much work is currently being done on developing enantioselective methods of synthesis, which yield only one of the two possible enantiomers. So important has enantioselective synthesis become that the 2001 Nobel Prize in Chemistry was awarded to three pioneers in the field: William S. Knowles of the Monsanto Co., K. Barry Sharpless of the Scripps Research Institute, and Ryoji Noyori of Nagoya University.
Several approaches to enantioselective synthesis have been developed, but the most efficient are those that use chiral catalysts to temporarily hold a substrate molecule in an unsymmetrical environment—the same strategy that nature uses when catalyzing reactions with chiral enzymes. While in that unsymmetrical environment, the substrate may be more open to reaction on one side than on another, leading to an excess of one enantiomeric product over another. As an analogy, think about picking up a coffee mug in your right hand to take a drink. The mug by itself is achiral, but as soon as you pick it up by the handle, it becomes chiral. One side of the mug now faces toward you so you can drink from it, but the other side faces away. The two sides are different, with one side much more accessible to you than the other.

Figure 10.11 A substance made from the tartaric acid found at the bottom of this wine vat catalyzes enantioselective reactions. (credit: “Pinot Noir fermentation in open vat” by Pipers Brook Vineyard Media/Flickr, CC BY 2.0)
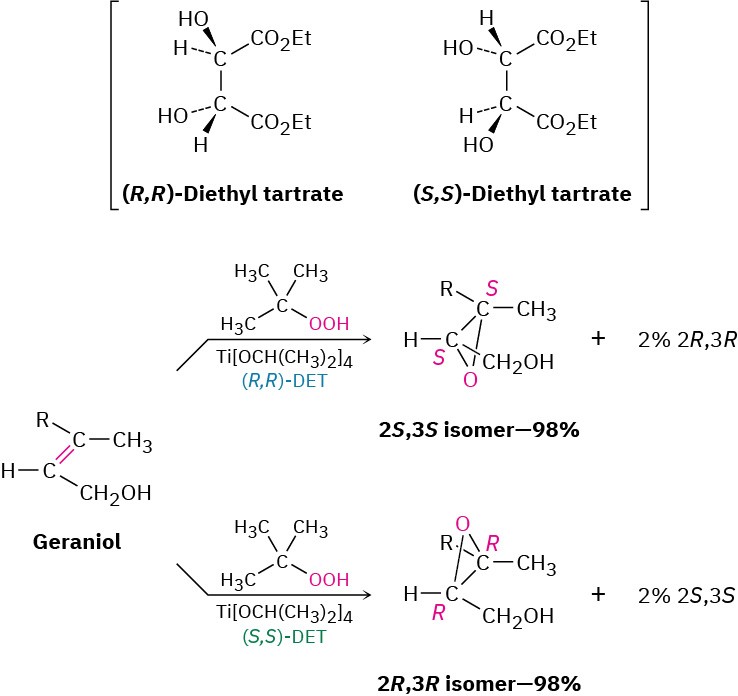
Among the thousands of enantioselective reactions now known, one of the most useful is the so-called Sharpless epoxidation, in which an allylic alcohol, such as geraniol, is treated with tert-butyl hydroperoxide, (CH3)3C–OOH, in the presence of titanium tetraisopropoxide and diethyl tartrate (DET) as a chiral auxiliary reagent. When (R,R) tartrate is used, geraniol is converted into its 2S,3S epoxide with 98% selectivity, whereas the use of (S,S) tartrate gives the 2R,3R epoxide enantiomer. We say that the major product in each case is formed with an enantiomeric excess of 96%, meaning that 4% of the product is racemic (2% 2S,3S plus 2% 2R,3R) and an extra 96% of a single enantiomer is formed. The mechanistic details by which the chiral catalyst works are a bit complex, although it appears that a chiral complex of two tartrate molecules with one titanium is involved.