11 Why This Chapter?
 Figure 11.1 The burning sensation produced by touching or eating chili peppers is due to capsaicin, a carboxylic acid derivative called an amide. (credit: modification of “Chillis” by Lucas Cobb/Flickr, CC BY 2.0)
Figure 11.1 The burning sensation produced by touching or eating chili peppers is due to capsaicin, a carboxylic acid derivative called an amide. (credit: modification of “Chillis” by Lucas Cobb/Flickr, CC BY 2.0)
Carboxylic acids are present in many industrial processes and most biological pathways and are the starting materials from which other acyl derivatives are made. Thus, an understanding of their properties and reactions is fundamental to understanding organic chemistry. We’ll look both at acids and at their close relatives, nitriles (RC≡N), and carboxylic acid derivatives.
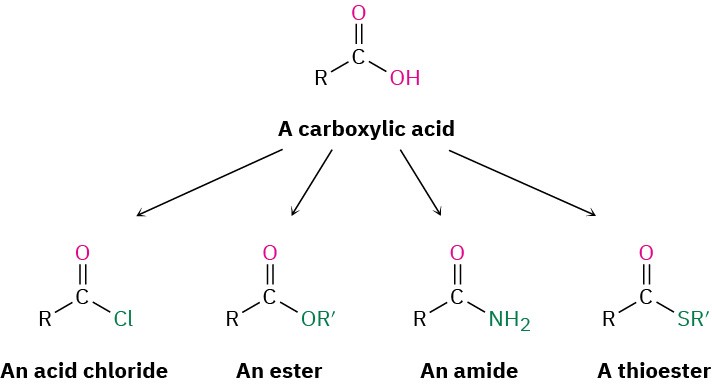
Carboxylic acids, RCO2H, occupy a central place among carbonyl compounds. Not only are they valuable in themselves, they also serve as starting materials for preparing numerous carboxylic acid derivatives such as acid chlorides, esters, amides, and thioesters. In addition, carboxylic acids are present in the majority of biological pathways.
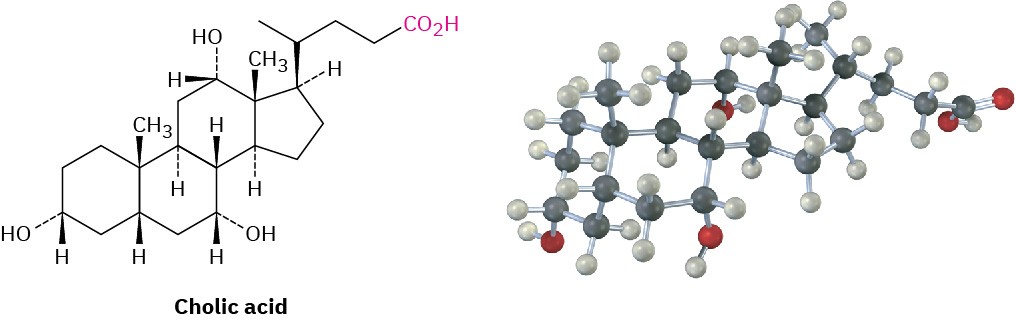
 A great many carboxylic acids are found in nature: acetic acid, CH3CO2H, is the chief organic component of vinegar; butanoic acid, CH3CH2CH2CO2H, is responsible for the rancid odor of sour butter; and hexanoic acid (caproic acid), CH3(CH2)4CO2H, is responsible for the unmistakable aroma of goats and dirty gym socks (the name comes from the Latin caper, meaning “goat”). Other examples are cholic acid, a major component of human bile, and long-chain aliphatic acids such as palmitic acid, CH3(CH2)14CO2H, a biological precursor of fats and vegetable oils.
A great many carboxylic acids are found in nature: acetic acid, CH3CO2H, is the chief organic component of vinegar; butanoic acid, CH3CH2CH2CO2H, is responsible for the rancid odor of sour butter; and hexanoic acid (caproic acid), CH3(CH2)4CO2H, is responsible for the unmistakable aroma of goats and dirty gym socks (the name comes from the Latin caper, meaning “goat”). Other examples are cholic acid, a major component of human bile, and long-chain aliphatic acids such as palmitic acid, CH3(CH2)14CO2H, a biological precursor of fats and vegetable oils.
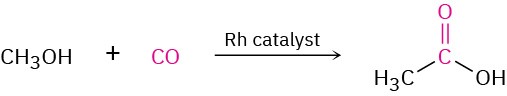
 Approximately 20 million tons of acetic acid is produced worldwide each year for a variety of purposes, including preparation of the vinyl acetate polymer used in paints and adhesives. About 20% of the acetic acid synthesized industrially is obtained by oxidation of acetaldehyde. Much of the remaining 80% is prepared by the rhodium-catalyzed reaction of methanol with carbon monoxide.
Approximately 20 million tons of acetic acid is produced worldwide each year for a variety of purposes, including preparation of the vinyl acetate polymer used in paints and adhesives. About 20% of the acetic acid synthesized industrially is obtained by oxidation of acetaldehyde. Much of the remaining 80% is prepared by the rhodium-catalyzed reaction of methanol with carbon monoxide.
 Carboxylic acid derivatives are among the most widely occurring of all molecules, both in laboratory chemistry and in biological pathways. Thus, a study of them and their primary reaction—nucleophilic acyl substitution—is fundamental to understanding organic chemistry. We’ll begin this chapter by first learning about carboxylic acid derivatives, and we’ll then explore the chemistry of acyl substitution reactions.
Carboxylic acid derivatives are among the most widely occurring of all molecules, both in laboratory chemistry and in biological pathways. Thus, a study of them and their primary reaction—nucleophilic acyl substitution—is fundamental to understanding organic chemistry. We’ll begin this chapter by first learning about carboxylic acid derivatives, and we’ll then explore the chemistry of acyl substitution reactions.
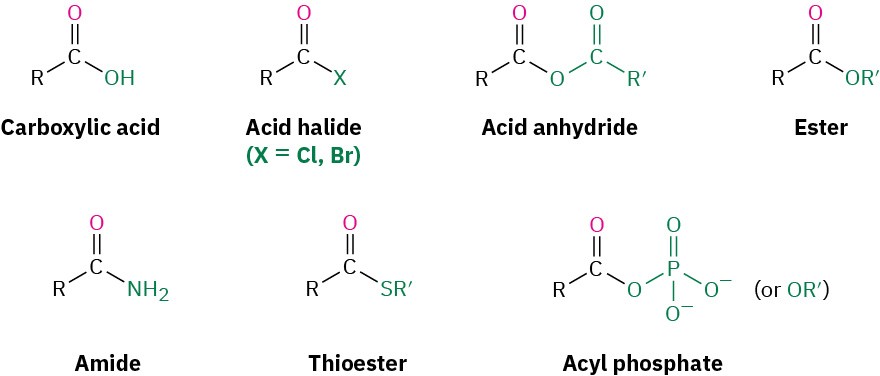
Closely related to the carboxylic acids and nitriles are the carboxylic acid derivatives, compounds in which an acyl group is bonded to an electronegative atom or substituent that can act as a leaving group in the nucleophilic acyl substitution reaction.
 Many kinds of acid derivatives are known, but we’ll be concerned primarily with four of the most common ones: acid halides, acid anhydrides, esters, and amides. Acid halides and acid anhydrides are used only in the laboratory, while esters and amides are common in both laboratory and biological chemistry. In addition, carboxylic acid derivatives called thioesters and acyl phosphates are encountered primarily in biological chemistry. Note the structural similarity between acid anhydrides and acyl phosphates.
Many kinds of acid derivatives are known, but we’ll be concerned primarily with four of the most common ones: acid halides, acid anhydrides, esters, and amides. Acid halides and acid anhydrides are used only in the laboratory, while esters and amides are common in both laboratory and biological chemistry. In addition, carboxylic acid derivatives called thioesters and acyl phosphates are encountered primarily in biological chemistry. Note the structural similarity between acid anhydrides and acyl phosphates.