Summary
Carboxylic acids are among the most useful building blocks for synthesizing other molecules, both in nature and in the laboratory. Thus, an understanding of their properties and reactions is fundamental to understanding biological chemistry. In this chapter, we’ve looked both at acids and at their close relatives, nitriles (RC≡N).
Carboxylic acids are named systematically by replacing the terminal –e of the corresponding alkane name with –oic acid. Like aldehydes and ketones, the carbonyl carbon atom is sp2-hybridized; like alcohols, carboxylic acids are associated through hydrogen- bonding and therefore have high boiling points.
The distinguishing characteristic of carboxylic acids is their acidity. Although weaker than mineral acids such as HCl, carboxylic acids dissociate much more readily than alcohols because the resultant carboxylate ions are stabilized by resonance between two equivalent forms.
Most carboxylic acids have pKa values near 5, but the exact pKa of a given acid depends on structure. Carboxylic acids substituted by electron-withdrawing groups are more acidic (have a lower pKa) because their carboxylate ions are stabilized. Carboxylic acids substituted by electron-donating groups are less acidic (have a higher pKa) because their carboxylate ions are destabilized. The extent of dissociation of a carboxylic acid in a buffered solution of a given pH can be calculated with the Henderson–Hasselbalch equation. Inside living cells, where the physiological pH = 7.3, carboxylic acids are entirely dissociated and exist as their carboxylate anions.
Methods of synthesis for carboxylic acids include (1) oxidation of alkylbenzenes, (2) oxidation of primary alcohols or aldehydes, and (3) hydrolysis of nitriles. General reactions of carboxylic acids include (1) loss of the acidic proton, (2) nucleophilic acyl substitution at the carbonyl group, and (3) reduction.
Nitriles are similar in some respects to carboxylic acids and are prepared either by SN2 reaction of an alkyl halide with cyanide ion or by dehydration of an amide. Nitriles undergo nucleophilic addition to the polar C≡N bond in the same way that carbonyl compounds do. The most important reactions of nitriles are their hydrolysis to carboxylic acids, reduction to primary amines, and reaction with Grignard reagents to yield ketones.
Carboxylic acid derivatives—compounds in which the –OH group of a carboxylic acid has been replaced by another substituent—are among the most widely occurring of all molecules and are involved in almost all biological pathways. In this chapter, we covered the chemistry necessary for understanding them and thus also necessary for understanding living organisms. Acid halides, acid anhydrides, esters, and amides are the most common such derivatives in the laboratory; thioesters and acyl phosphates are common in biological molecules.
The chemistry of carboxylic acid derivatives is dominated by the nucleophilic acyl substitution reaction. Mechanistically, these substitutions take place by addition of a nucleophile to the polar carbonyl group of the acid derivative to give a tetrahedral intermediate, followed by expulsion of a leaving group.
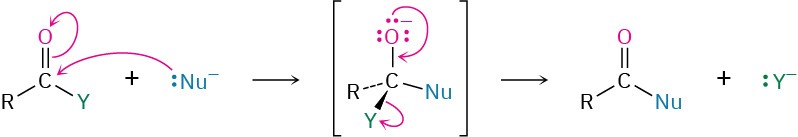
The reactivity of an acid derivative toward substitution depends both on the steric environment near the carbonyl group and on the electronic nature of the substituent, Y. The reactivity order is acid halide > acid anhydride > thioester > ester > amide.
The most common reactions of carboxylic acid derivatives are substitution by water to yield an acid (hydrolysis), by an alcohol to yield an ester (alcoholysis), by an amine to yield an amide (aminolysis), by hydride ion to yield an alcohol (reduction), and by an organomagnesium halide to yield an alcohol (Grignard reaction).
Step-growth polymers, such as polyamides and polyesters, are prepared by reactions between difunctional molecules. Polyamides (nylons) are formed by reaction between a diacid and a diamine; polyesters are formed from a diacid and a diol.

