12.4 Synthesis of Amines
Reduction of Nitriles, Amides, and Nitro Compounds
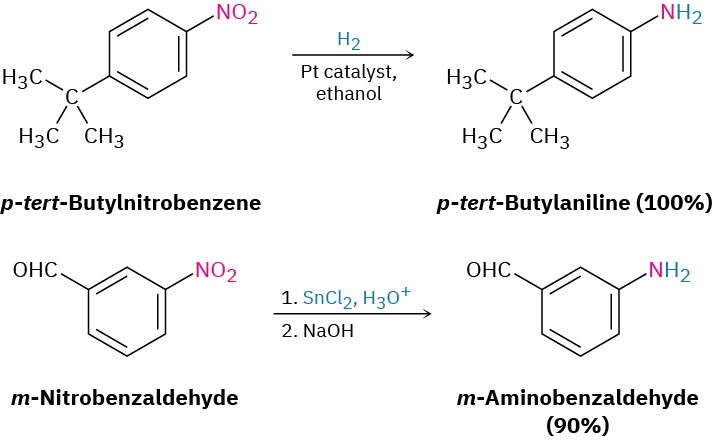
We’ve already seen in Section 11.10 and Section 11.11 how amines can be prepared by reduction of nitriles and amides with LiAlH4. The two-step sequence of SN2 displacement with CN– followed by reduction thus converts an alkyl halide into a primary alkylamine having an additional carbon atom. Amide reduction converts carboxylic acids and their derivatives into amines with the same number of carbon atoms.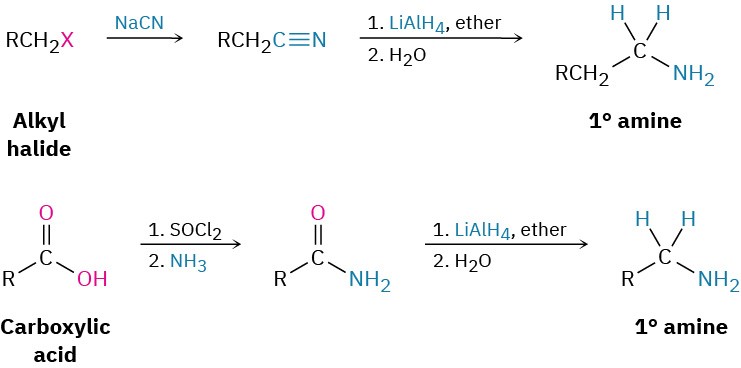 Arylamines are usually prepared by nitration of an aromatic starting material, followed by reduction of the nitro group (Section 8.4). The reduction step can be carried out in many different ways, depending on the circumstances. Catalytic hydrogenation over platinum works well but is often incompatible with the presence elsewhere in the molecule of other reducible groups, such as C=C bonds or carbonyl groups. Iron, zinc, tin, and tin(II) chloride (SnCl2) are also effective when used in acidic aqueous solution. Tin(II) chloride is particularly mild and is often used when other reducible functional groups are present.
Arylamines are usually prepared by nitration of an aromatic starting material, followed by reduction of the nitro group (Section 8.4). The reduction step can be carried out in many different ways, depending on the circumstances. Catalytic hydrogenation over platinum works well but is often incompatible with the presence elsewhere in the molecule of other reducible groups, such as C=C bonds or carbonyl groups. Iron, zinc, tin, and tin(II) chloride (SnCl2) are also effective when used in acidic aqueous solution. Tin(II) chloride is particularly mild and is often used when other reducible functional groups are present.
 Problem 12.7
Problem 12.7
Propose structures for either a nitrile or an amide that might be a precursor of each of the following amines:
(a) CH3CH2CH2NH2
(b) (CH3CH2CH2)2NH
(c) Benzylamine, C6H5CH2NH2
(d) N-Ethylaniline
SN2 Reactions of Alkyl Halides
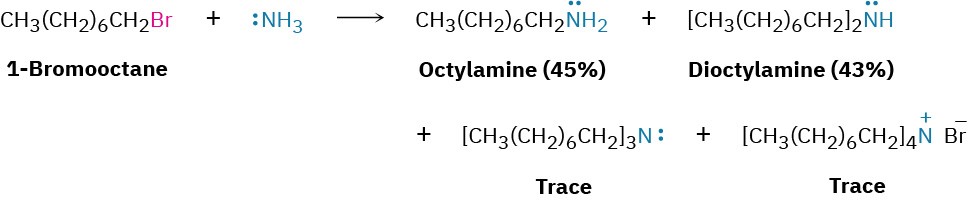
Ammonia and other amines are good nucleophiles in SN2 reactions. As a result, the simplest method of alkylamine synthesis is by SN2 alkylation of ammonia or an alkylamine with an alkyl halide. If ammonia is used, a primary amine results; if a primary amine is used, a secondary amine results; and so on. Even tertiary amines react rapidly with alkyl halides to yield quaternary ammonium salts, R4N+ X–.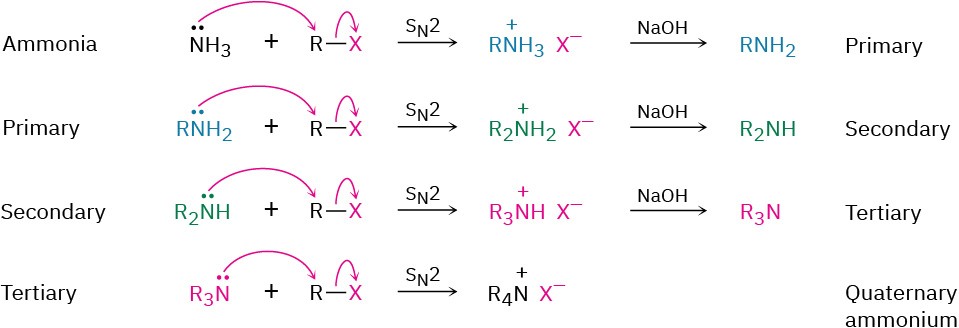 Unfortunately, these reactions don’t stop cleanly after a single alkylation has occurred. Because ammonia and primary amines have similar reactivity, the initially formed monoalkylated substance often undergoes further reaction to yield a mixture of products. Even secondary and tertiary amines undergo further alkylation, although to a lesser extent. For example, treatment of 1-bromooctane with a twofold excess of ammonia leads to a mixture containing only 45% octylamine. A nearly equal amount of dioctylamine is produced by double alkylation, along with smaller amounts of trioctylamine and tetraoctylammonium bromide.
Unfortunately, these reactions don’t stop cleanly after a single alkylation has occurred. Because ammonia and primary amines have similar reactivity, the initially formed monoalkylated substance often undergoes further reaction to yield a mixture of products. Even secondary and tertiary amines undergo further alkylation, although to a lesser extent. For example, treatment of 1-bromooctane with a twofold excess of ammonia leads to a mixture containing only 45% octylamine. A nearly equal amount of dioctylamine is produced by double alkylation, along with smaller amounts of trioctylamine and tetraoctylammonium bromide.
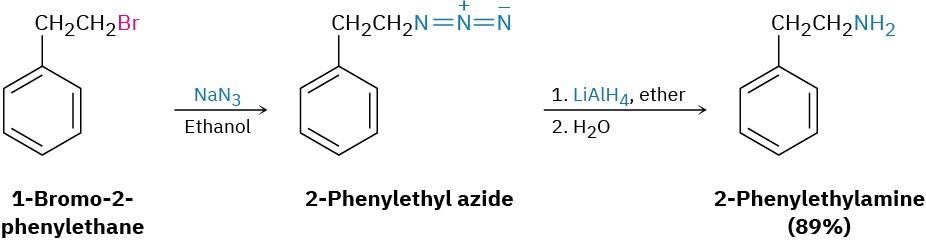
 A better method for preparing primary amines is to use azide ion, N3–, rather than ammonia, as the nucleophile for SN2 reaction with a primary or secondary alkyl halide. The product is an alkyl azide, which is not nucleophilic, so overalkylation can’t occur. Subsequent reduction of the alkyl azide with LiAlH4 then leads to the desired primary amine. Although this method works well, low-molecular-weight alkyl azides are explosive and must be handled carefully.
A better method for preparing primary amines is to use azide ion, N3–, rather than ammonia, as the nucleophile for SN2 reaction with a primary or secondary alkyl halide. The product is an alkyl azide, which is not nucleophilic, so overalkylation can’t occur. Subsequent reduction of the alkyl azide with LiAlH4 then leads to the desired primary amine. Although this method works well, low-molecular-weight alkyl azides are explosive and must be handled carefully.
 Problem 12.8
Problem 12.8
Show two methods for the synthesis of dopamine, a neurotransmitter involved in regulation of the central nervous system. Use any alkyl halide needed.
Reductive Amination of Aldehydes and Ketones
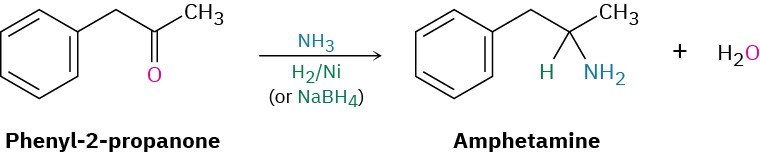
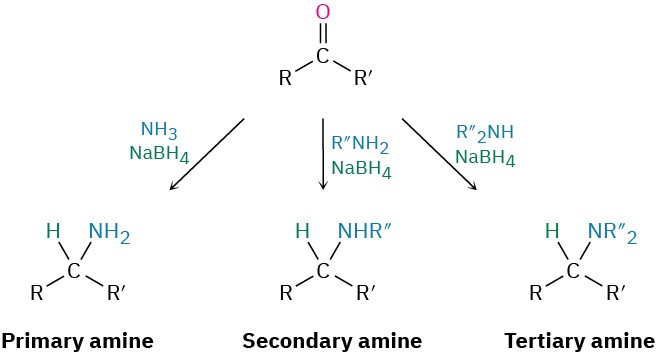
Amines can be synthesized in a single step by treatment of an aldehyde or ketone with ammonia or an amine in the presence of a reducing agent, a process called reductive amination. For example, amphetamine, a central nervous system stimulant, is prepared commercially by reductive amination of phenyl-2-propanone with ammonia using hydrogen gas over a nickel catalyst as the reducing agent. In the laboratory, either NaBH4 or the related NaBH(OAc)3 is commonly used (OAc = acetate).
 Reductive amination takes place by the pathway shown in Figure 12.5. An imine intermediate is first formed by a nucleophilic addition reaction (Section 10.8), and the C═N bond of the imine is then reduced to the amine, much as the C═O bond of a ketone can be reduced to an alcohol.
Reductive amination takes place by the pathway shown in Figure 12.5. An imine intermediate is first formed by a nucleophilic addition reaction (Section 10.8), and the C═N bond of the imine is then reduced to the amine, much as the C═O bond of a ketone can be reduced to an alcohol.
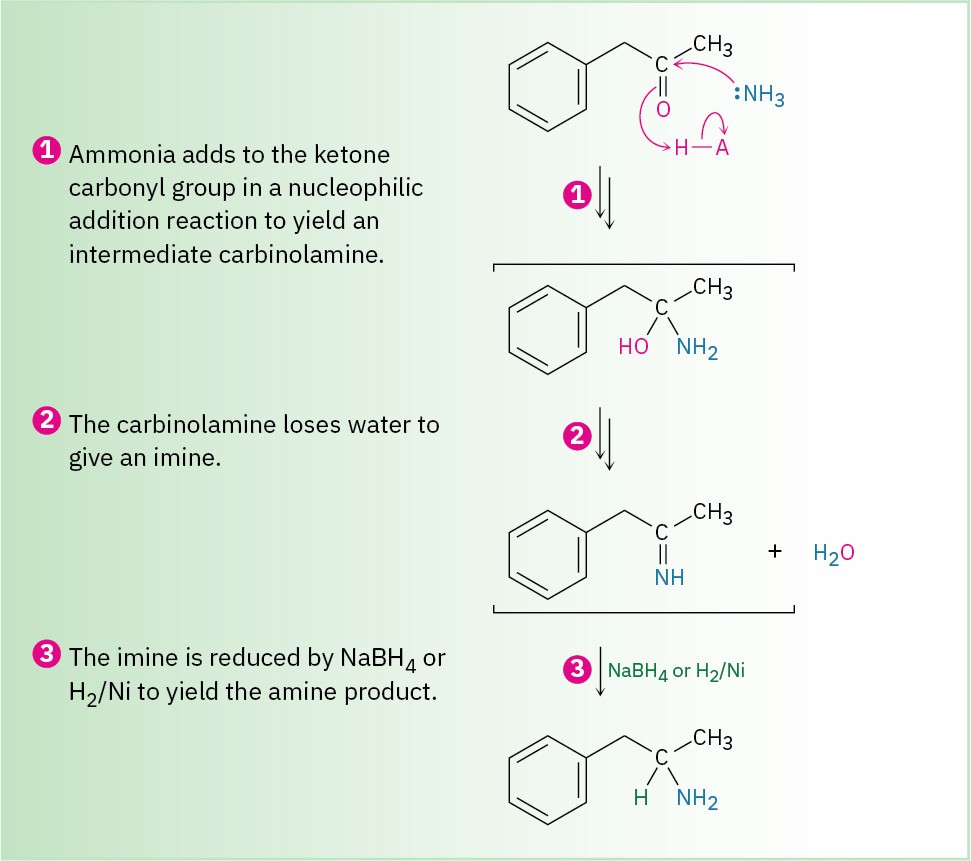
Figure 12.5 MECHANISM: Mechanism for reductive amination of a ketone to yield an amine.
Details of the imine-forming step are shown in Figure 10.7.Ammonia, primary amines, and secondary amines can all be used in the reductive amination reaction, yielding primary, secondary, and tertiary amines, respectively.
 Reductive aminations also occur in various biological pathways. In the biosynthesis of the amino acid proline, for instance, glutamate 5-semialdehyde undergoes internal imine formation to give 1-pyrrolinium-5-carboxylate, which is then reduced by nucleophilic addition of hydride ion to the C═N bond. Reduced nicotinamide adenine dinucleotide, NADH, acts as the biological reducing agent.
Reductive aminations also occur in various biological pathways. In the biosynthesis of the amino acid proline, for instance, glutamate 5-semialdehyde undergoes internal imine formation to give 1-pyrrolinium-5-carboxylate, which is then reduced by nucleophilic addition of hydride ion to the C═N bond. Reduced nicotinamide adenine dinucleotide, NADH, acts as the biological reducing agent.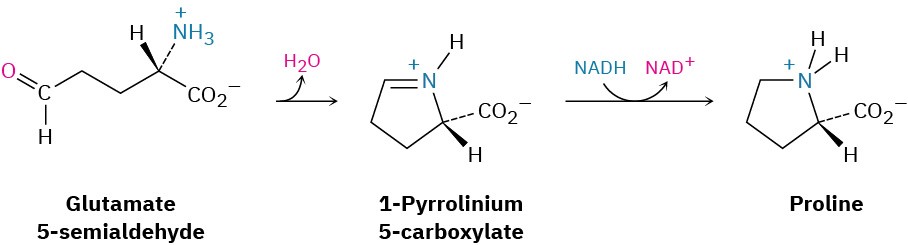
Worked Example 12.1: Using a Reductive Amination Reaction
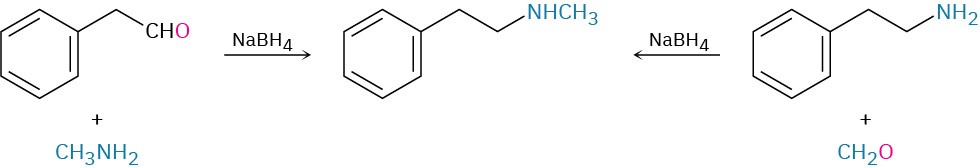
How might you prepare N-methyl-2-phenylethylamine using a reductive amination reaction?

Strategy
Look at the target molecule, and identify the groups attached to nitrogen. One of the groups must be derived from the aldehyde or ketone component, and the other must be derived from the amine component. In the case of N-methyl-2-phenylethylamine, two combinations can lead to the product: phenylacetaldehyde plus methylamine or formaldehyde plus 2- phenylethylamine. It’s usually better to choose the combination with the simpler amine component—methylamine in this case—and to use an excess of that amine as reactant.
Solution
 Problem 12.9
Problem 12.9
How might the following amines be prepared using reductive amination reactions? Show all precursors if more than one is possible.
(a)
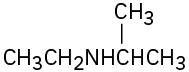
(b)
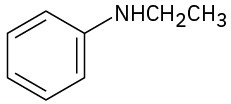
(c)
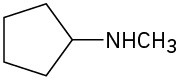
Problem 12.10
How could you prepare the following amine using a reductive amination reaction?