2.10 Stability of Cycloalkanes: Ring Strain
Chemists in the late 1800s knew that cyclic molecules existed, but the limitations on ring size were unclear. Although numerous compounds containing five-membered and six-membered rings were known, smaller and larger ring sizes had not been prepared, despite many attempts.
A theoretical interpretation of this observation was proposed in 1885 by Adolf von Baeyer, who suggested that small and large rings might be unstable due to angle strain—the strain induced in a molecule when bond angles are forced to deviate from the ideal 109° tetrahedral value. Baeyer based his suggestion on the simple geometric notion that a three-membered ring (cyclopropane) should be an equilateral triangle with bond angles of 60° rather than 109°, a four-membered ring (cyclobutane) should be a square with bond angles of 90°, a five-membered ring should be a regular pentagon with bond angles of 108°, and so on. Continuing this argument, large rings should be strained by having bond angles that are much greater than 109°.
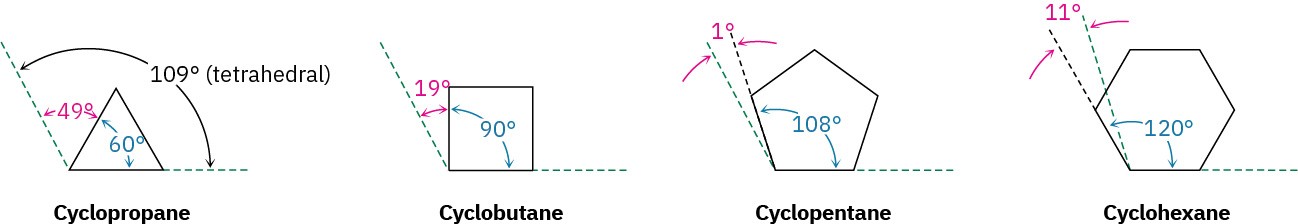
Baeyer’s theory is wrong for the simple reason that he assumed all cycloalkanes to be flat. In fact, as we’ll see in the next section, most cycloalkanes are not flat; instead, they adopt puckered three-dimensional conformations that allow bond angles to be nearly tetrahedral. As a result, angle strain occurs only in three- and four-membered rings, which have little flexibility. For most ring sizes, particularly the medium-ring (C7–C11) cycloalkanes, torsional strain caused by H ⟷ H eclipsing interactions at adjacent carbons (Section 2.7) and steric strain caused by the repulsion between nonbonded atoms that approach too closely (Section 2.8) are the most important factors. Thus, three kinds of strain contribute to the overall energy of a cycloalkane.
- Angle strain—the strain due to expansion or compression of bond angles
- Torsional strain—the strain due to eclipsing of bonds between neighboring atoms
- Steric strain—the strain due to repulsive interactions when atoms approach each other too closely
Problem 2.25
cis-1,2-Dimethylcyclopropane has more strain than trans-1,2-dimethylcyclopropane. How can you account for this difference? Which of the two compounds is more stable?

