3.4 Pasteur’s Discovery of Enantiomers
Little was done to build on Biot’s discovery of optical activity until 1848, when Louis Pasteur began work on a study of crystalline tartaric acid salts derived from wine. On crystallizing a concentrated solution of sodium ammonium tartrate below 28 °C, Pasteur made the surprising observation that two distinct kinds of crystals were obtained.
Furthermore, the two kinds of crystals were nonsuperimposable mirror images and were related in the same way that a right hand is related to a left hand.
Working carefully with tweezers, Pasteur was able to separate the crystals into two piles, one of “right-handed” crystals and one of “left-handed” crystals, like those shown in Figure 3.7. Although the original sample, a 50 : 50 mixture of right and left, was optically inactive, solutions of the crystals from each of the sorted piles were optically active and their specific rotations were equal in magnitude but opposite in sign.
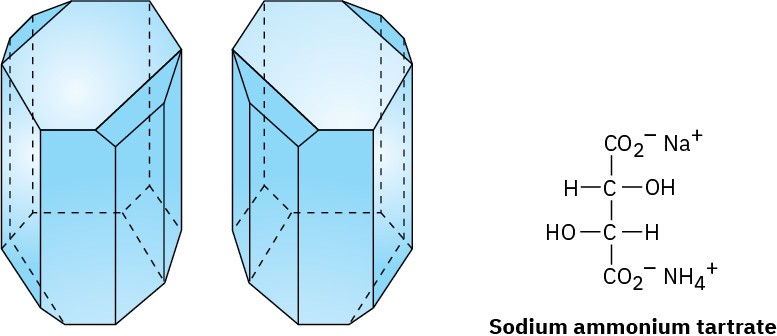 Figure 3.7 Drawings of sodium ammonium tartrate crystals taken from Pasteur’s original sketches. One of the crystals is dextrorotatory in solution, and the other is levorotatory.
Figure 3.7 Drawings of sodium ammonium tartrate crystals taken from Pasteur’s original sketches. One of the crystals is dextrorotatory in solution, and the other is levorotatory.
Pasteur was far ahead of his time. Although the structural theory of Kekulé had not yet been proposed, Pasteur explained his results by speaking of the molecules themselves, saying, “There is no doubt that [in the dextro tartaric acid] there exists an asymmetric arrangement having a nonsuperimposable image. It is no less certain that the atoms of the levo acid have precisely the inverse asymmetric arrangement.” Pasteur’s vision was extraordinary, for it was not until 25 years later that his ideas regarding asymmetric carbon atoms were confirmed.
Today, we would describe Pasteur’s work by saying that he had discovered enantiomers. Enantiomers, also called optical isomers, have identical physical properties, such as melting point and boiling point, but differ in the direction in which their solutions rotate plane-polarized light.

