3.7 Meso Compounds
Let’s look at another example (Section 3.4) of a compound with more than one chirality center: the tartaric acid used by Pasteur. The four stereoisomers can be drawn as follows:
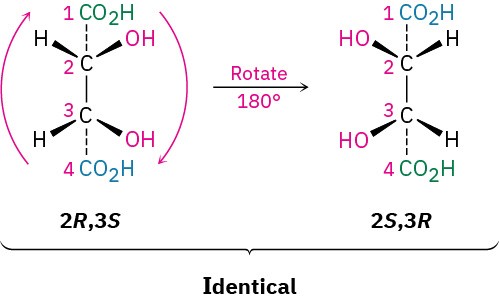
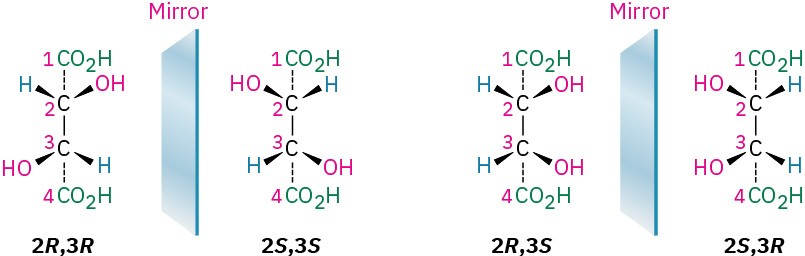 The 2R,3R and 2S,3S structures are nonsuperimposable mirror images and therefore represent a pair of enantiomers. A close look at the 2R,3S and 2S,3R structures, however, shows that they are superimposable, and thus identical, as can be seen by rotating one structure 180°.
The 2R,3R and 2S,3S structures are nonsuperimposable mirror images and therefore represent a pair of enantiomers. A close look at the 2R,3S and 2S,3R structures, however, shows that they are superimposable, and thus identical, as can be seen by rotating one structure 180°.
 The 2R,3S and 2S,3R structures are identical because the molecule has a plane of symmetry and is therefore achiral. The symmetry plane cuts through the C2–C3 bond, making one half of the molecule a mirror image of the other half (Figure 3.12). Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Such compounds, which are achiral, yet contain chirality centers, are called meso compounds (me-zo). Thus, tartaric acid exists in three stereoisomeric forms: two enantiomers and one meso form.
The 2R,3S and 2S,3R structures are identical because the molecule has a plane of symmetry and is therefore achiral. The symmetry plane cuts through the C2–C3 bond, making one half of the molecule a mirror image of the other half (Figure 3.12). Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Such compounds, which are achiral, yet contain chirality centers, are called meso compounds (me-zo). Thus, tartaric acid exists in three stereoisomeric forms: two enantiomers and one meso form.
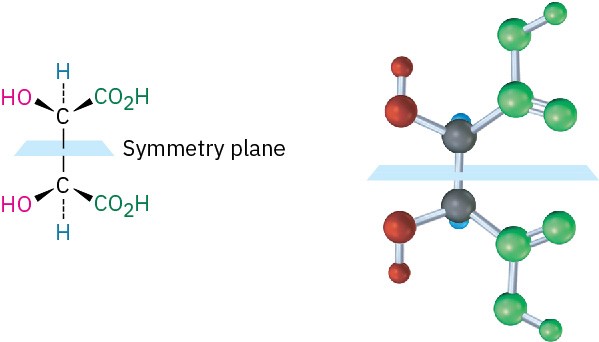 Figure 3.12 A symmetry plane through the C2–C3 bond of meso-tartaric acid makes the molecule achiral.
Figure 3.12 A symmetry plane through the C2–C3 bond of meso-tartaric acid makes the molecule achiral.
Some physical properties of the three stereoisomers are listed in Table 3.3. The (+)- and (−)-tartaric acids have identical melting points, solubilities, and densities, but they differ in the sign of their rotation of plane-polarized light. The meso isomer, by contrast, is diastereomeric with the (+) and (−) forms. It has no mirror-image relationship to (+)- and (−)-tartaric acids, is a different compound altogether, and thus has different physical properties.
Table 3.3 Some Properties of the Stereoisomers of Tartaric Acid Some Properties of the Stereoisomers of Tartaric Acid
|
Melting point (°C) |
[α]D |
Density (g/cm3) |
Solubility at 20 °C (g/100 mL H2O) |
|
|
(+) |
168–170 |
+12 |
1.7598 |
139.0 |
|
(−) |
168–170 |
−12 |
1.7598 |
139.0 |
|
Meso |
146–148 |
0 |
1.6660 |
125.0 |
Worked Example 3.5: Distinguishing Chiral Compounds from Meso Compounds
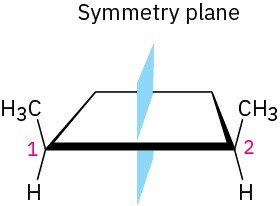
Does cis-1,2-dimethylcyclobutane have any chirality centers? Is it chiral?
Strategy
To see whether a chirality center is present, look for a carbon atom bonded to four different groups. To see whether the molecule is chiral, look for the presence or absence of a symmetry plane. Not all molecules with chirality centers are chiral overall—meso compounds are an exception.
Solution
A look at the structure of cis-1,2-dimethylcyclobutane shows that both methyl-bearing ring carbons (C1 and C2) are chirality centers. Overall, though, the compound is achiral because there is a symmetry plane bisecting the ring between C1 and C2. Thus, the molecule is a meso compound.
 Problem 3.14
Problem 3.14
Which of the following structures represent meso compounds?
(a)
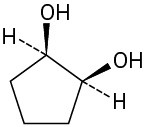
(b)
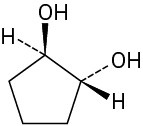
(c)
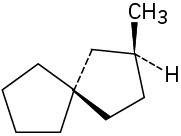
(d)
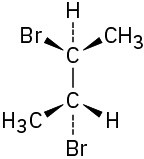
Problem 3.15
Which of the following have a meso form? (Recall that the –ol suffix refers to an alcohol, ROH.)
(a) 2,3-Butanediol
(b) 2,3-Pentanediol
(c) 2,4-Pentanediol

