Appendix E: Bond Dissociation Energies for Some Organic Compounds
Table E1. Bond Dissociation Energies (D) in Kj/mol of Some Organic Compounds
| Bond | D (kJ/mol) | Bond | D (kJ/mol) | Bond | D (kJ/mol) |
| H-H | 436 | (CH3)2CH-H | 410 | C2H5–CH3 | 370 |
| H-F | 570 | (CH3)2CH-Cl | 354 | (CH3)2CH-CH3 | 369 |
| H-Cl | 431 | (CH3)2CH-Br | 299 | (CH3)3C-CH3 | 363 |
| H-Br | 366 | (CH3)3C-H | 400 | H2C=CH-CH3 | 426 |
| H-I | 298 | (CH3)3C-Cl | 352 | H2C=CHCH2–CH3 | 318 |
| Cl-Cl | 242 | (CH3)3C-Br | 293 | H2C=CH2 | 728 |
| Br-Br | 194 | (CH3)3C-I | 227 | 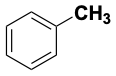 |
427 |
| I-I | 152 | H2C=CH-H | 464 | 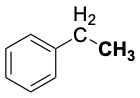 |
325 |
| CH3–H | 439 | H2C=CH-Cl | 396 | 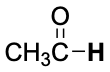 |
374 |
| CH3–Cl | 350 | H2C=CHCH2–H | 369 | HO-H | 497 |
| CH3–Br | 294 | H2C=CHCH2–Cl | 298 | HO-OH | 211 |
| CH3–I | 239 | 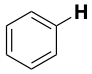 |
472 | CH3O-H | 440 |
| CH3–OH | 385 | 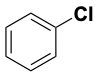 |
400 | CH3S-H | 366 |
| CH3–NH2 | 386 | 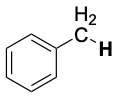 |
375 | C2H5O-H | 441 |
| C2H5–H | 421 | 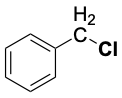 |
300 | 352 | |
| C2H5–Cl | 352 | 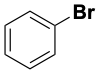 |
336 | CH3CH2O-CH3 | 355 |
| C2H5–Br | 293 |  |
464 | NH2–H | 450 |
| C2H5–I | 233 | HC≡C-H | 558 | H-CN | 528 |
| C2H5-OH | 391 | CH3–CH3 | 377 |

