3.10 Chirality in Nature and Chiral Environments
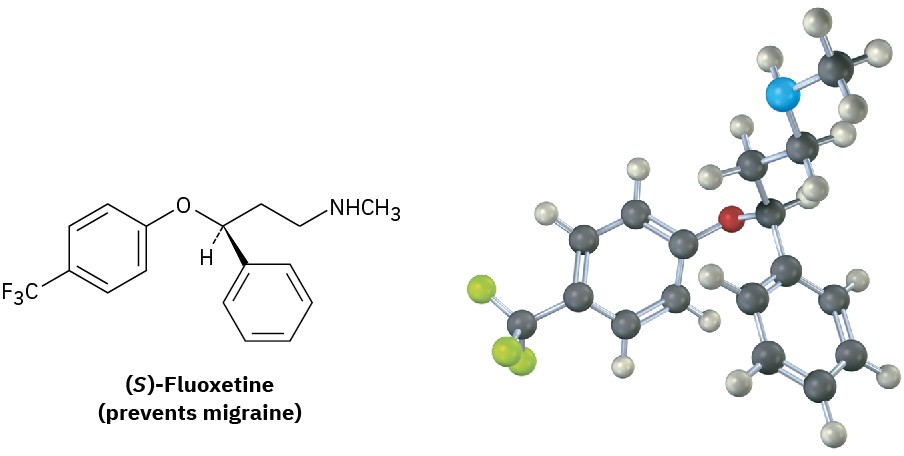
Although the different enantiomers of a chiral molecule have the same physical properties, they usually have different biological properties. For example, a change in chirality can affect the biological properties of many drugs, such as fluoxetine, a heavily prescribed medication sold under the trade name Prozac. Racemic fluoxetine is an effective antidepressant but has no activity against migraine. The pure S enantiomer, however, works remarkably well in preventing migraine. Other examples of how chirality affects biological properties are given at the end of this chapter.
 Why do different enantiomers have different biological properties? To have a biological effect, a substance typically must fit into an appropriate receptor that has a complementary shape. But because biological receptors are chiral, only one enantiomer of a chiral substrate can fit, just as only a right hand can fit into a right-handed glove. The mirror-image enantiomer will be a misfit, like a left hand in a right-handed glove. A representation of the interaction between a chiral molecule and a chiral biological receptor is shown in Figure 3.16: one enantiomer fits the receptor perfectly, but the other does not.
Why do different enantiomers have different biological properties? To have a biological effect, a substance typically must fit into an appropriate receptor that has a complementary shape. But because biological receptors are chiral, only one enantiomer of a chiral substrate can fit, just as only a right hand can fit into a right-handed glove. The mirror-image enantiomer will be a misfit, like a left hand in a right-handed glove. A representation of the interaction between a chiral molecule and a chiral biological receptor is shown in Figure 3.16: one enantiomer fits the receptor perfectly, but the other does not.
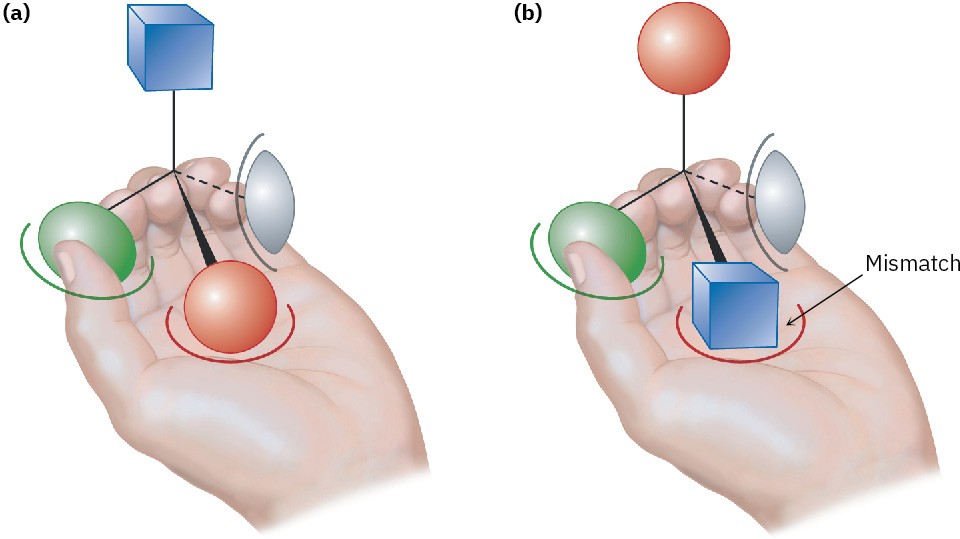
Figure 3.16 Interaction of a chiral object with a chiral receptor. A left hand interacts with a chiral object, much as a biological receptor interacts with a chiral molecule. (a) One enantiomer fits into the hand perfectly: green thumb, red palm, and gray pinkie finger, with the blue substituent exposed. (b) The other enantiomer, however, can’t fit into the hand. When the green thumb and gray pinkie finger interact appropriately, the palm holds a blue substituent rather than a red one, with the red substituent exposed.
The hand-in-glove fit of a chiral substrate into a chiral receptor is relatively straightforward, but it’s less obvious how a prochiral substrate can undergo a selective reaction. Take the reaction of ethanol with NAD+ catalyzed by yeast alcohol dehydrogenase. As we saw at the end of Section 3.11, this reaction occurs with exclusive removal of the pro- R hydrogen from ethanol and with addition only to the Re face of the NAD+ carbon.
We can understand this result by imagining that the chiral enzyme receptor again has three binding sites, as in Figure 3.16. When green and gray substituents of a prochiral substrate are held appropriately, however, only one of the two red substituents—say, the pro-S one—is also held while the other, pro-R, substituent is exposed for reaction.
We describe the situation by saying that the receptor provides a chiral environment for the substrate. In the absence of a chiral environment, the two red substituents are chemically identical, but in the presence of a chiral environment, they are chemically distinctive (Figure 3.17a). The situation is similar to what happens when you pick up a coffee mug. By itself, the mug has a plane of symmetry and is achiral. When you pick up the mug, however, your hand provides a chiral environment so that one side becomes much more accessible and easier to drink from than the other (Figure 3.17b).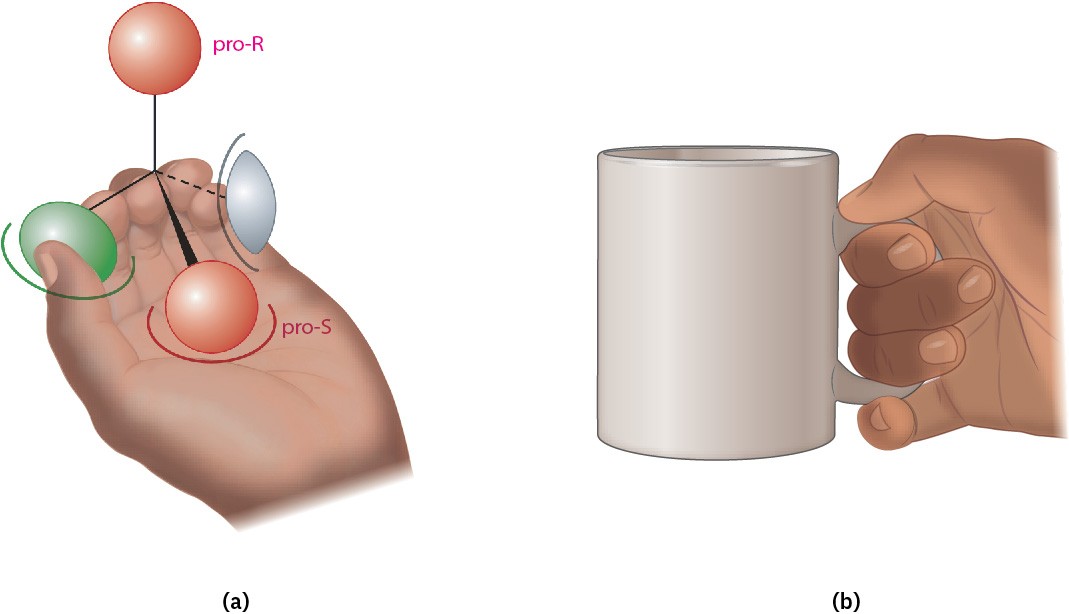 Figure 3.17 (a) When a prochiral molecule is held in a chiral environment, the two seemingly identical substituents are distinguishable. (b) Similarly, when an achiral coffee mug is held in the chiral environment of your hand, it’s much easier to drink from one side than the other because the two sides of the mug are now distinguishable.
Figure 3.17 (a) When a prochiral molecule is held in a chiral environment, the two seemingly identical substituents are distinguishable. (b) Similarly, when an achiral coffee mug is held in the chiral environment of your hand, it’s much easier to drink from one side than the other because the two sides of the mug are now distinguishable.

