Appendix B | Acidity Constants for Some Organic Compounds
Acidity Constants for Some Organic Compounds
|
Compound |
pKa |
| CH3SO3H | -1.8 |
| CH(NO2)3 | 0.1 |
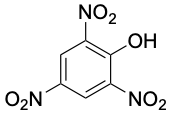 |
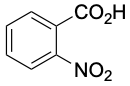
0.3 |
| CCl3CO2H | 0.5 |
| CF3CO2H | 0.5 |
| CBr3CO2H | 0.7 |
| HO2CC≡CCO2H | 1.2; 2.5 |
| HO2CCO2H | 1.2; 3.7 |
| CHCl2CO2H | 1.3 |
| CH2(NO2)CO2H | 1.3 |
| HC≡CCO2H | 1.9 |
| (Z) HO2CCH=CHCO2H | 1.9; 6.3 |
|
|
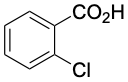
2.4 |
| CH3COCO2H | 2.4 |
| NCCH2CO2H | 2.5 |
| CH3C≡CCO2H | 2.6 |
| CH2FCO2H | 2.7 |
| CH2ClCO2H | 2.8 |
| HO2CCH2CO2H | 2.8; 5.6 |
| CH2BrCO2H | 2.9 |
|
|
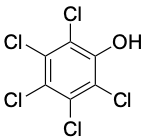
3.0 |
| Compound | pKa |
|
|
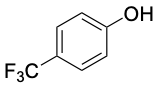
3.0 |
| CH2ICO2H | 3.2 |
| CHOCO2H | 3.2 |
|
|
3.4 |
|
|
3.5 |
| HSCH2CO2H | 3.5; 10.2 |
| CH2(NO2)2 | 3.6 |
| CH3OCH2CO2H | 3.6 |
| CH3COCH2CO2H | 3.6 |
| HOCH2CO2H | 3.7 |
| HCO2H | 3.7 |
|
|
3.8 |
|
|
4.0 |
| CH2BrCH2CO2H | 4.0 |
|
|
4.1 |
|
|
4.2 |
| H2C=CHCO2H | 4.2 |
| HO2CCH2CH2CO2H | 4.3; 5.7 |
| HO2CCH2CH2CH2CO2H | 4.3; 5.4 |
|
|
4.5 |
| H2C=CH(CH3)CO2H | 4.7 |
| CH3CO2H | 4.8 |
| CH3CH2CO2H | 4.8 |
| Compound | pKa |
| (CH3)3CCO2H | 5.0 |
| CH3COCH2NO2 | 5.1 |
|
|
5.3 |
| O2NCH2CO2CH3 | 5.8 |
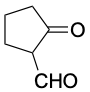 |
5.8 |
|
|
6.2 |
|
|
6.6 |
| HCO3H | 7.1 |
|
|
7.2 |
| (CH3)2CHNO2 | 7.7 |
|
|
7.8 |
| CH3CO3H | 8.2 |
|
|
8.5 |
| CH3CH2NO2 | 8.5 |
|
|
8.7 |
| CH3COCH2COCH3 | 9.0 |
|
|
9.3; 11.1 |
|
|
9.3; 12.6 |
| Compound | pKa |
| 9.4 | |
| 9.9; 11.5 | |
| 9.9 | |
| CH3COCH2SOCH3 | 10.0 |
| 10.3 | |
| CH3NO2 | 10.3 |
| CH3SH | 10.3 |
| CH3COCH2CO2CH3 | 10.6 |
| CH3COCHO | 11.0 |
| CH2(CN)2 | 11.2 |
| CCl3CH2OH | 12.2 |
| Glucose | 12.3 |
| (CH3)2C=NOH | 12.4 |
| CH2(CO2CH3)2 | 12.9 |
| CHCl2CH2OH | 12.9 |
| CH2(OH)2 | 13.3 |
| HOCH2CH(OH)CH2OH | 14.1 |
| CH2ClCH2OH | 14.3 |
| 15.0 | |
| 15.4 | |
| CH3OH | 15.5 |
| H2C=CHCH2OH | 15.5 |
| CH3CH2OH | 16.0 |
| CH3CH2CH2OH | 16.1 |
| CH3COCH2Br | 16.1 |
| 16.7 | |
| CH3CHO | 17 |
| Compound | pKa |
| (CH3)2CHCHO | 17 |
| (CH3)2CHOH | 17.1 |
| (CH3)3COH | 18.0 |
| CH3COCH3 | 19.3 |
| 23 | |
| CH3CO2CH2CH3 | 25 |
| HC≡CH | 25 |
| CH3CN | 25 |
| CH3SO2CH3 | 28 |
| (C6H5)3CH | 32 |
| (C6H5)2CH2 | 34 |
| CH3SOCH3 | 35 |
| NH3 | 36 |
| CH3CH2NH2 | 36 |
| (CH3CH2)2NH | 40 |
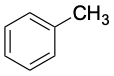 |
41 |
| 43 | |
| H2C=CH2 | 44 |
| CH4 | ~60 |
An acidity list covering more than 5000 organic compounds has been published: E.P. Serjeant and B. Dempsey (eds.), “Ionization Constants of Organic Acids in Aqueous Solution,” IUPAC Chemical Data Series No. 23, Pergamon Press, Oxford, 1979.