11.11 Chemistry of Nitriles
Nitriles are analogous to carboxylic acids in that both have a carbon atom with three bonds to an electronegative atom and both contain a π bond. Thus, some reactions of nitriles and carboxylic acids are similar. Both kinds of compounds are electrophiles, for instance, and both undergo nucleophilic addition reactions.
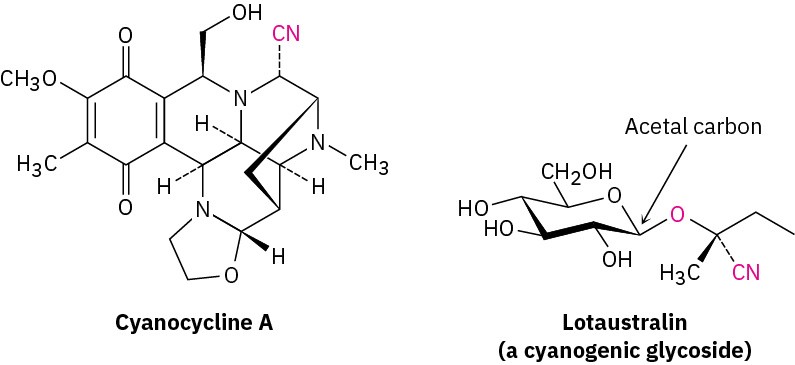
 Nitriles occur infrequently in living organisms, although several hundred examples are known. Cyanocycline A, for instance, has been isolated from the bacterium Streptomyces lavendulae and was found to have both antimicrobial and antitumor activity. In addition, more than 1000 compounds called cyanogenic glycosides are known. Derived primarily from plants, cyanogenic glycosides contain a sugar with an acetal carbon, one oxygen of which is bonded to a nitrile-bearing carbon (sugar–O–C–CN). On hydrolysis with aqueous acid, the acetal is cleaved (Section 10.9), generating a cyanohydrin (HO–C–CN), which releases hydrogen cyanide. It’s thought that the primary function of cyanogenic glycosides is to protect the plant by poisoning any animal foolish enough to eat it. Lotaustralin from the cassava plant is an example.
Nitriles occur infrequently in living organisms, although several hundred examples are known. Cyanocycline A, for instance, has been isolated from the bacterium Streptomyces lavendulae and was found to have both antimicrobial and antitumor activity. In addition, more than 1000 compounds called cyanogenic glycosides are known. Derived primarily from plants, cyanogenic glycosides contain a sugar with an acetal carbon, one oxygen of which is bonded to a nitrile-bearing carbon (sugar–O–C–CN). On hydrolysis with aqueous acid, the acetal is cleaved (Section 10.9), generating a cyanohydrin (HO–C–CN), which releases hydrogen cyanide. It’s thought that the primary function of cyanogenic glycosides is to protect the plant by poisoning any animal foolish enough to eat it. Lotaustralin from the cassava plant is an example.
Preparation of Nitriles
The simplest method of nitrile preparation is the SN2 reaction of CN– with a primary or secondary alkyl halide, as discussed in Section 11.4. Another method for preparing nitriles is by dehydration of a primary amide, RCONH2. Thionyl chloride is often used for this reaction, although other dehydrating agents such as POCl3 also work.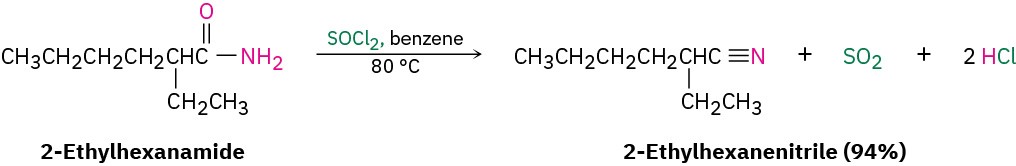
Reactions of Nitriles
Like a carbonyl group, a nitrile group is strongly polarized and has an electrophilic carbon atom. Nitriles therefore react with nucleophiles to yield sp2-hybridized imine anions in a reaction analogous to the formation of a sp3-hybridized alkoxide ion by nucleophilic addition to a carbonyl group.
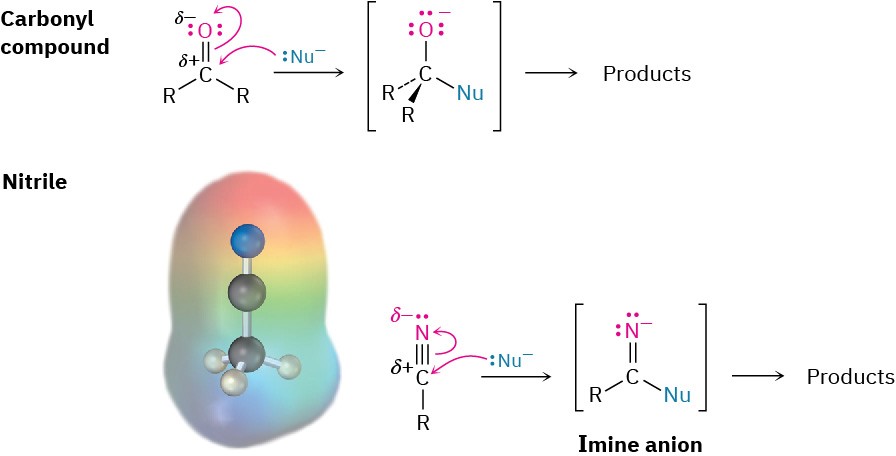 Some general reactions of nitriles are shown in Figure 11.11.
Some general reactions of nitriles are shown in Figure 11.11.
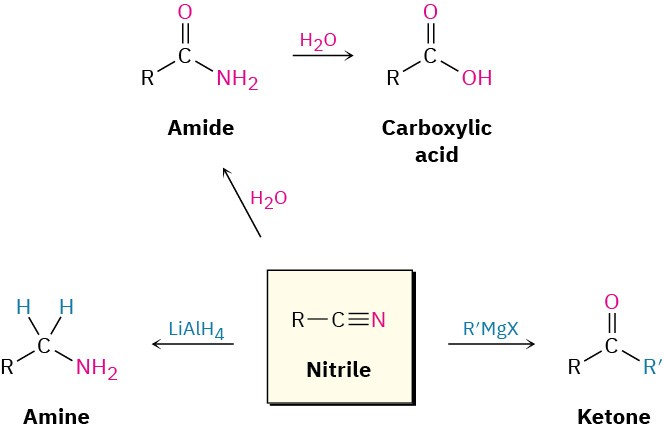
Figure 11.11 Some reactions of nitriles.
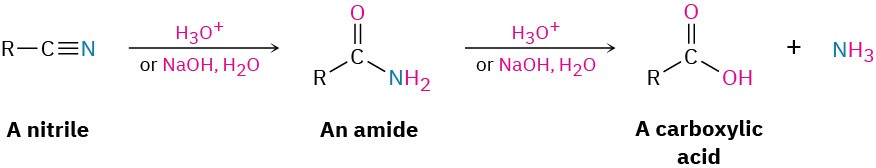
Hydrolysis: Conversion of Nitriles into Carboxylic Acids
Among the most useful reactions of nitriles is their hydrolysis to yield first an amide and then a carboxylic acid plus ammonia or an amine. The reaction occurs in either basic or acidic aqueous solution:
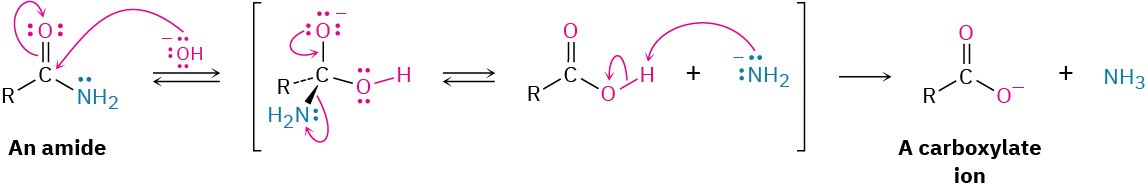
 As shown in Figure 11.12, base-catalyzed nitrile hydrolysis involves nucleophilic addition of hydroxide ion to the polar C≡N bond to give an imine anion in a process similar to the nucleophilic addition to a polar C═O bond to give an alkoxide anion. Protonation then gives a hydroxy imine, which tautomerizes (Section 9.4) to an amide in a step similar to the tautomerization of an enol to a ketone. Further hydrolysis gives a carboxylate ion.
As shown in Figure 11.12, base-catalyzed nitrile hydrolysis involves nucleophilic addition of hydroxide ion to the polar C≡N bond to give an imine anion in a process similar to the nucleophilic addition to a polar C═O bond to give an alkoxide anion. Protonation then gives a hydroxy imine, which tautomerizes (Section 9.4) to an amide in a step similar to the tautomerization of an enol to a ketone. Further hydrolysis gives a carboxylate ion.
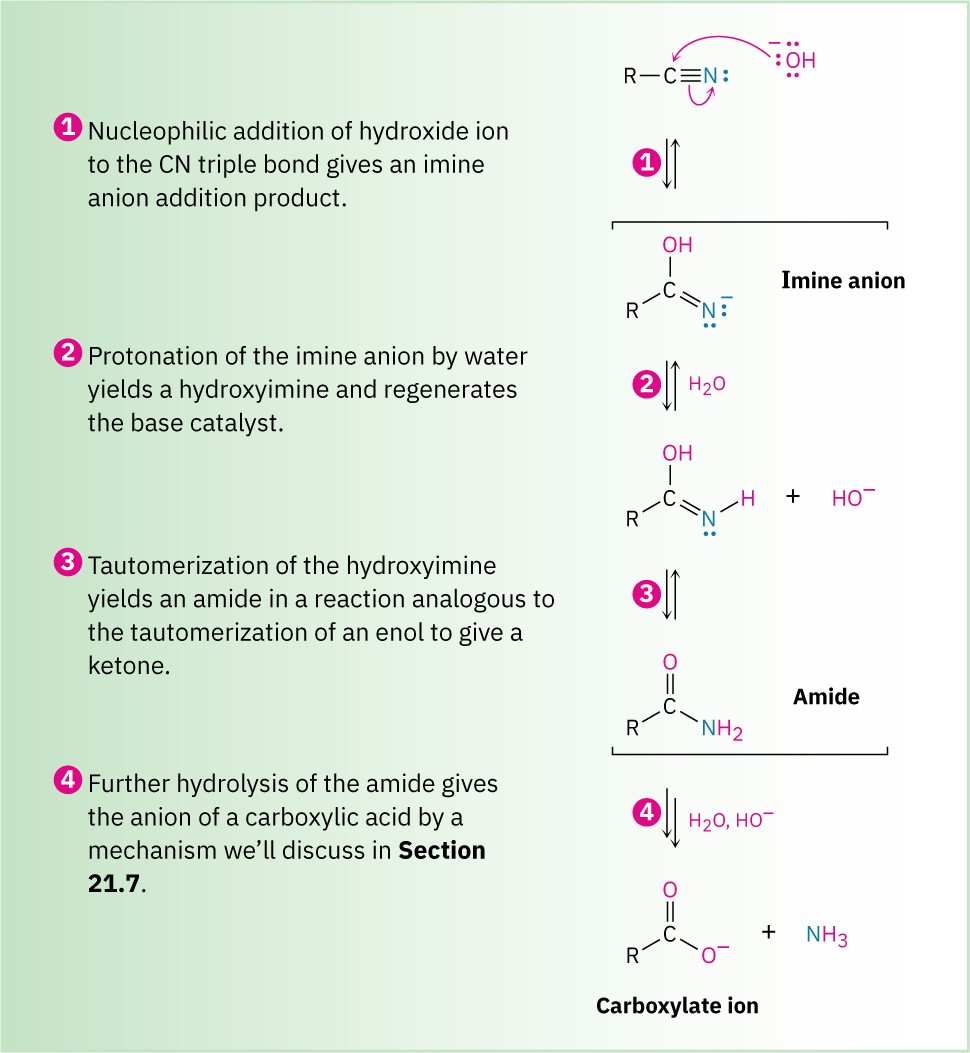
Figure 11.12 MECHANISM: Mechanism for the basic hydrolysis of a nitrile to yield an amide, which is then hydrolyzed further to a carboxylic acid anion.
The further hydrolysis of the amide intermediate takes place by a nucleophilic addition of hydroxide ion to the amide carbonyl group, which yields a tetrahedral alkoxide ion.
Expulsion of amide ion, NH2−, as leaving group gives the carboxylate ion, thereby driving the reaction toward the products. Subsequent acidification in a separate step yields the carboxylic acid. We’ll look at this process in more detail in Section 11.10.
Reduction: Conversion of Nitriles into Amines
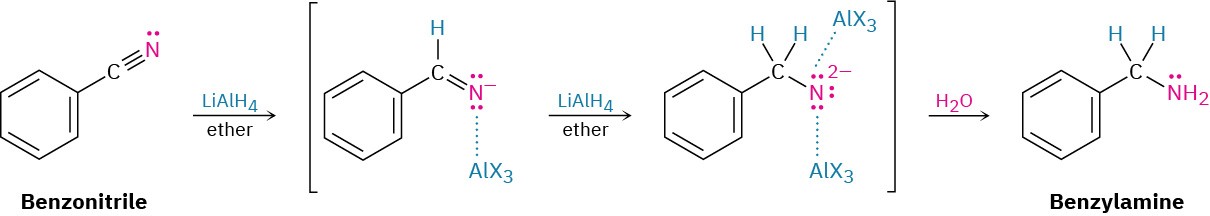
Reduction of a nitrile with LiAlH4 gives a primary amine, RNH2. The reaction occurs by nucleophilic addition of hydride ion to the polar C≡N bond, yielding an imine anion, which still contains a C═N bond and therefore undergoes a second nucleophilic addition of hydride to give a dianion. Both monoanion and dianion intermediates are undoubtedly stabilized by Lewis acid–base complexation to an aluminum species, facilitating the second addition that would otherwise be difficult. Protonation of the dianion by addition of water in a subsequent step gives the amine.
Reaction of Nitriles with Grignard Reagents
Grignard reagents add to a nitrile to give an intermediate imine anion that is hydrolyzed by addition of water to yield a ketone. The mechanism of the hydrolysis is the exact reverse of imine formation (see Figure 10.8).
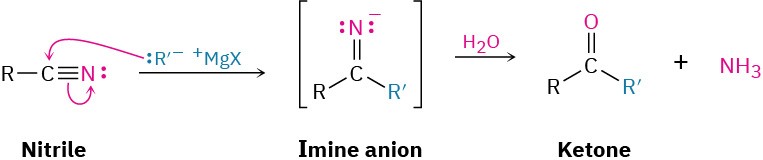 This reaction is similar to the reduction of a nitrile to an amine, except that only one nucleophilic addition occurs rather than two and the attacking nucleophile is a carbanion (R:–) rather than a hydride ion. For example:
This reaction is similar to the reduction of a nitrile to an amine, except that only one nucleophilic addition occurs rather than two and the attacking nucleophile is a carbanion (R:–) rather than a hydride ion. For example:
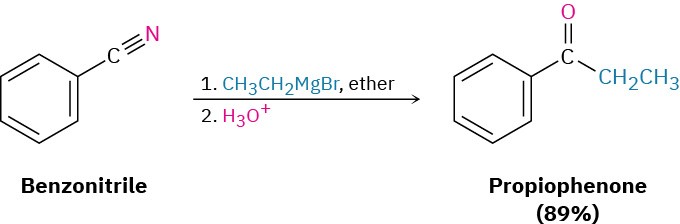
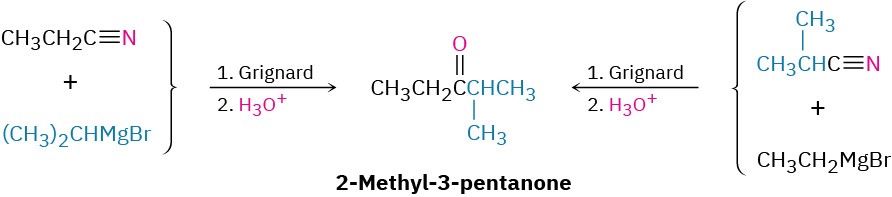
Worked Example 11.5: Synthesizing a Ketone from a Nitrile
How would you prepare 2-methyl-3-pentanone from a nitrile?

Strategy
A ketone results from the reaction between a Grignard reagent and a nitrile, with the C≡N carbon of the nitrile becoming the carbonyl carbon. Identify the two groups attached to the carbonyl carbon atom in the product. One will come from the Grignard reagent and the other will come from the nitrile.
Solution
There are two possibilities.
 Problem 11.29
Problem 11.29
How would you prepare the following carbonyl compounds from a nitrile?
(a)
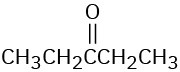
(b)
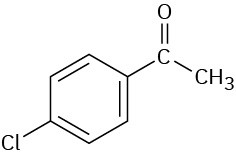
Problem 11.30
How would you prepare 1-phenyl-2-butanone, C6H5CH2COCH2CH3, from benzyl bromide, C6H5CH2Br? More than one step is needed.