8.7 An Explanation of Substituent Effects
Activating and Deactivating Effects
What makes a group either activating or deactivating? The common characteristic of all activating groups is that they donate electrons to the ring, thereby making the ring more electron-rich, stabilizing the carbocation intermediate, and lowering the activation energy for its formation. Conversely, the common characteristic of all deactivating groups is that they withdraw electrons from the ring, thereby making the ring more electron-poor, destabilizing the carbocation intermediate, and raising the activation energy for its formation.
 Compare the electrostatic potential maps of benzaldehyde (deactivated), chlorobenzene (weakly deactivated), and phenol (activated) with that of benzene. As shown in Figure 8.12, the ring is more positive (yellow-green) when an electron-withdrawing group such as –CHO or –Cl is present and more negative (red) when an electron-donating group such as –OH is present.
Compare the electrostatic potential maps of benzaldehyde (deactivated), chlorobenzene (weakly deactivated), and phenol (activated) with that of benzene. As shown in Figure 8.12, the ring is more positive (yellow-green) when an electron-withdrawing group such as –CHO or –Cl is present and more negative (red) when an electron-donating group such as –OH is present.
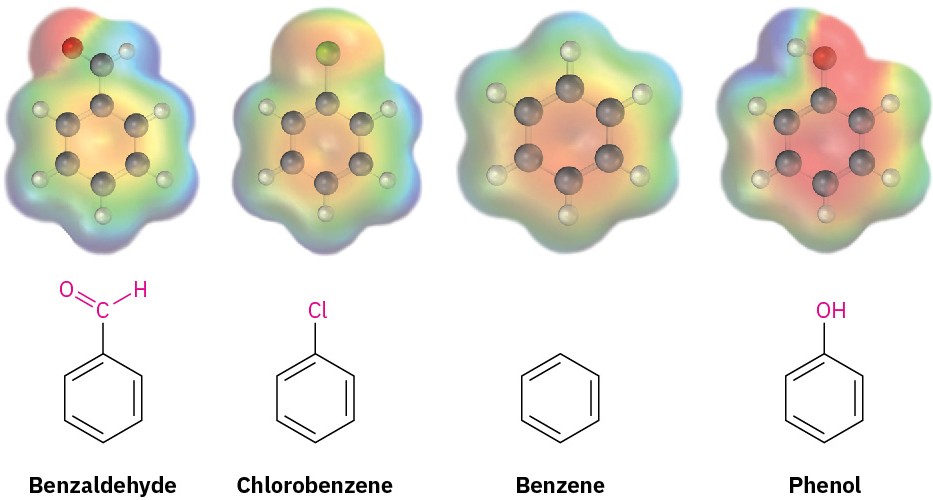
Figure 8.12 Electrostatic potential maps of benzene and several substituted benzenes show that an electron-withdrawing group (–CHO or –Cl) makes the ring more electron-poor, while an electron-donating group (–OH) makes the ring more electron-rich.
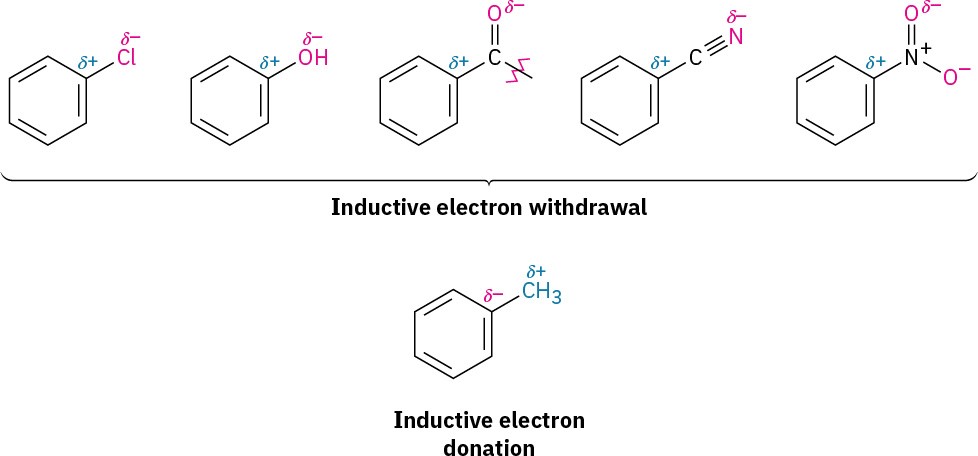
The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. As we saw in Section 1.12, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Halogens, hydroxyl groups, carbonyl groups, cyano groups, and nitro groups inductively withdraw electrons through the σ bond linking the substituent to a benzene ring. This effect is most pronounced in halobenzenes and phenols, in which the electronegative atom is directly attached to the ring, but is also significant in carbonyl compounds, nitriles, and nitro compounds, in which the electronegative atom is farther removed. Alkyl groups, on the other hand, inductively donate electrons. This is the same hyperconjugative donating effect that causes alkyl substituents to stabilize alkenes (Section 4.3) and carbocations (Section 5.2).
A resonance effect is the withdrawal or donation of electrons through a π bond due to the overlap of a p orbital on the substituent with a p orbital on the aromatic ring. Carbonyl, cyano, and nitro substituents, for example, withdraw electrons from the aromatic ring by resonance. The π electrons flow from the ring to the substituent, leaving a positive charge in the ring. Note that substituents with an electron-withdrawing resonance effect have the general structure –Y=Z, where the Z atom is more electronegative than Y.
Conversely, halogen, hydroxyl, alkoxyl (–OR), and amino substituents donate electrons to the aromatic ring by resonance. Lone-pair electrons flow from the substituents to the ring, placing a negative charge on the ring. Substituents with an electron-donating resonance effect have the general structure –Y, where the Y atom has a lone pair of electrons available for donation to the ring.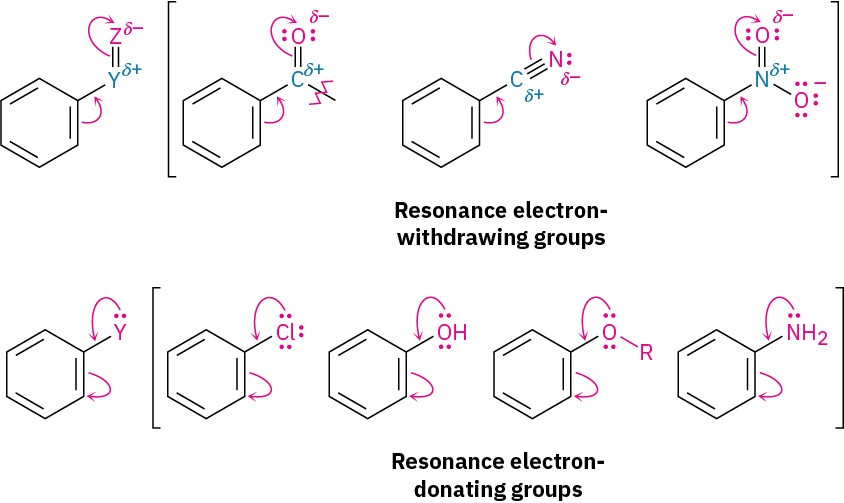 One further point: inductive effects and resonance effects don’t necessarily act in the same direction. Halogen, hydroxyl, alkoxyl, and amino substituents, for instance, have electron- withdrawing inductive effects because of the electronegativity of the –X, –O, or –N atom bonded to the aromatic ring but have electron-donating resonance effects because of the lone-pair electrons on those –X, –O, or –N atoms. When the two effects act in opposite directions, the stronger effect dominates. Thus, hydroxyl, alkoxyl, and amino substituents are activators because their stronger electron-donating resonance effect outweighs their weaker electron-withdrawing inductive effect. Halogens, however, are deactivators because their stronger electron-withdrawing inductive effect outweighs their weaker electron-donating resonance effect.
One further point: inductive effects and resonance effects don’t necessarily act in the same direction. Halogen, hydroxyl, alkoxyl, and amino substituents, for instance, have electron- withdrawing inductive effects because of the electronegativity of the –X, –O, or –N atom bonded to the aromatic ring but have electron-donating resonance effects because of the lone-pair electrons on those –X, –O, or –N atoms. When the two effects act in opposite directions, the stronger effect dominates. Thus, hydroxyl, alkoxyl, and amino substituents are activators because their stronger electron-donating resonance effect outweighs their weaker electron-withdrawing inductive effect. Halogens, however, are deactivators because their stronger electron-withdrawing inductive effect outweighs their weaker electron-donating resonance effect.
Problem 8.10
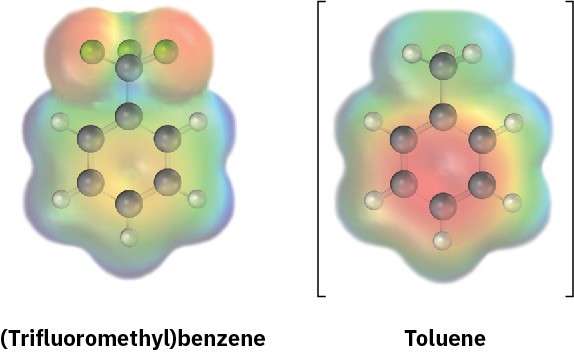
An electrostatic potential map of (trifluoromethyl)benzene, C6H5CF3, is shown. Would you expect (trifluoromethyl)benzene to be more reactive or less reactive than toluene toward electrophilic substitution? Explain.
Ortho- and Para-Directing Activators: Alkyl Groups
Inductive and resonance effects account not only for reactivity but also for the orientation of electrophilic aromatic substitutions. Take alkyl groups, for instance, which have an electron-donating inductive effect and are ortho and para directors. The results of toluene nitration are shown in Figure 8.13.
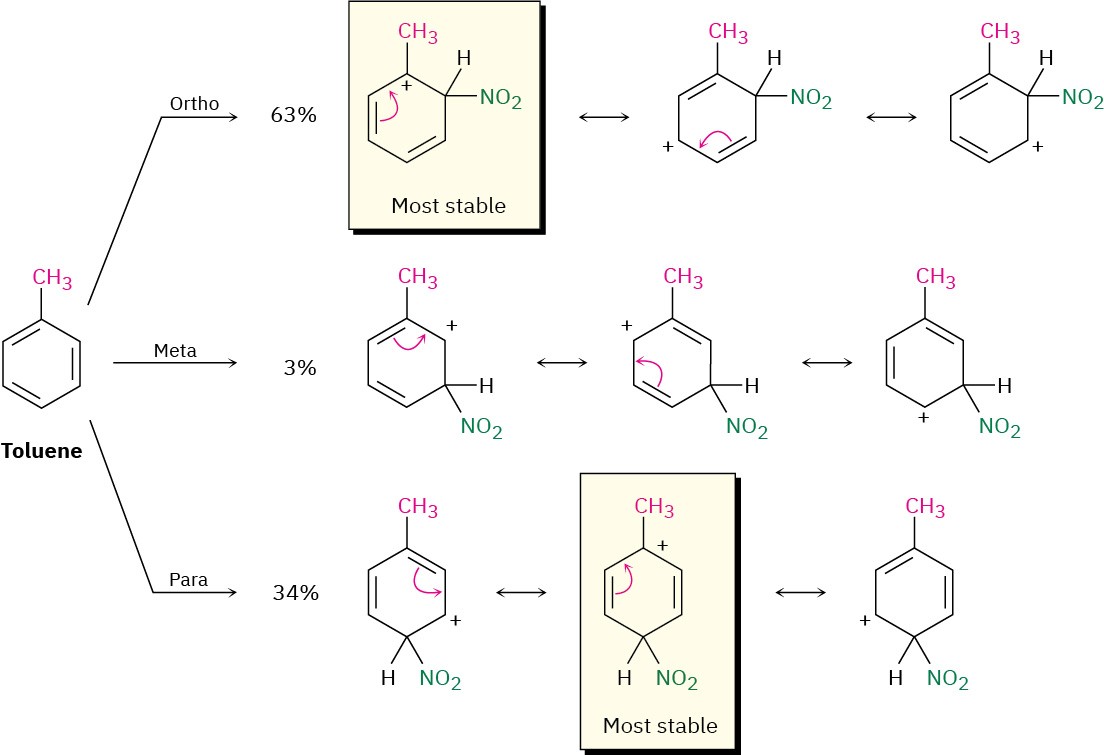 Figure 8.13 Carbocation intermediates in the nitration of toluene. Ortho and para intermediates are more stable than the meta intermediate because the positive charge is on a tertiary carbon rather than a secondary carbon.
Figure 8.13 Carbocation intermediates in the nitration of toluene. Ortho and para intermediates are more stable than the meta intermediate because the positive charge is on a tertiary carbon rather than a secondary carbon.
Nitration of toluene might occur either ortho, meta, or para to the methyl group, giving the three carbocation intermediates shown in in Figure 8.13. Although all three intermediates are resonance-stabilized, the ortho and para intermediates are more stabilized than the meta intermediate. For both the ortho and para reactions, but not for the meta reaction, a resonance form places the positive charge directly on the methyl-substituted carbon, where it is in a tertiary position and can be stabilized by the electron-donating inductive effect of the methyl group. The ortho and para intermediates are thus lower in energy than the meta intermediate and form faster.
Ortho- and Para-Directing Activators: OH and NH2
Hydroxyl, alkoxyl, and amino groups are also ortho–para activators, but for a different reason than for alkyl groups. As described earlier in this section, hydroxyl, alkoxyl, and amino groups have a strong, electron-donating resonance effect that outweighs a weaker electron-withdrawing inductive effect. When phenol is nitrated, for instance, reaction can occur either ortho, meta, or para to the –OH group, giving the carbocation intermediates shown in Figure 8.14. The ortho and para intermediates are more stable than the meta intermediate because they have more resonance forms, including one particularly favorable form that allows the positive charge to be stabilized by electron donation from the substituent oxygen atom. The intermediate from the meta reaction has no such stabilization.
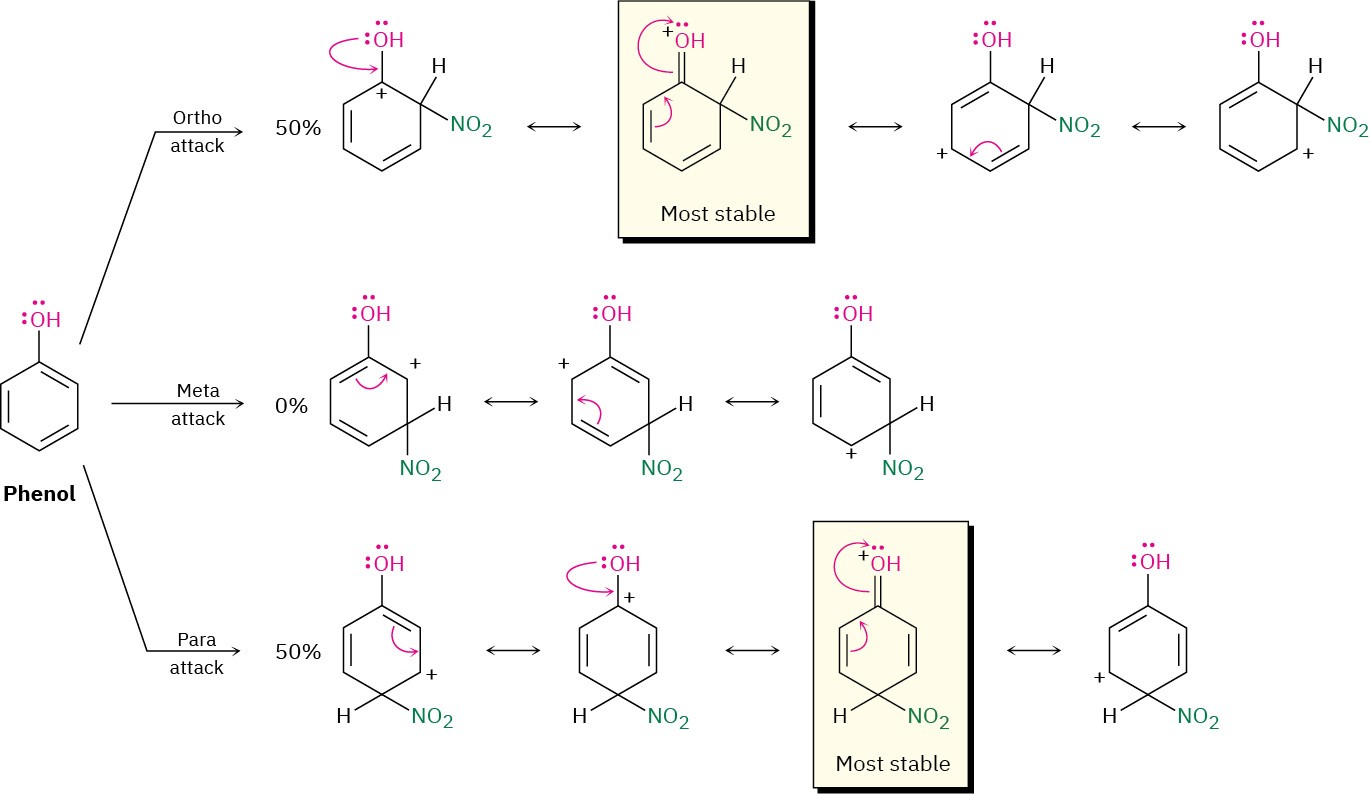 Figure 8.14 Carbocation intermediates in the nitration of phenol. The ortho and para intermediates are more stable than the meta intermediate because they have more resonance forms, including one particularly favorable form that involves electron donation from the oxygen atom.
Figure 8.14 Carbocation intermediates in the nitration of phenol. The ortho and para intermediates are more stable than the meta intermediate because they have more resonance forms, including one particularly favorable form that involves electron donation from the oxygen atom.
Problem 8.11
Acetanilide is less reactive than aniline toward electrophilic substitution. Explain.
Ortho- and Para-Directing Deactivators: Halogens
Halogens are deactivating because their stronger electron-withdrawing inductive effect outweighs their weaker electron-donating resonance effect. Although weak, that electron- donating resonance effect is nevertheless felt only at the ortho and para positions and not at the meta position (Figure 8.15). Thus, a halogen substituent can stabilize the positive charge of the carbocation intermediates from ortho and para reaction in the same way that hydroxyl and amino substituents can. The meta intermediate, however, has no such stabilization and is therefore formed more slowly.
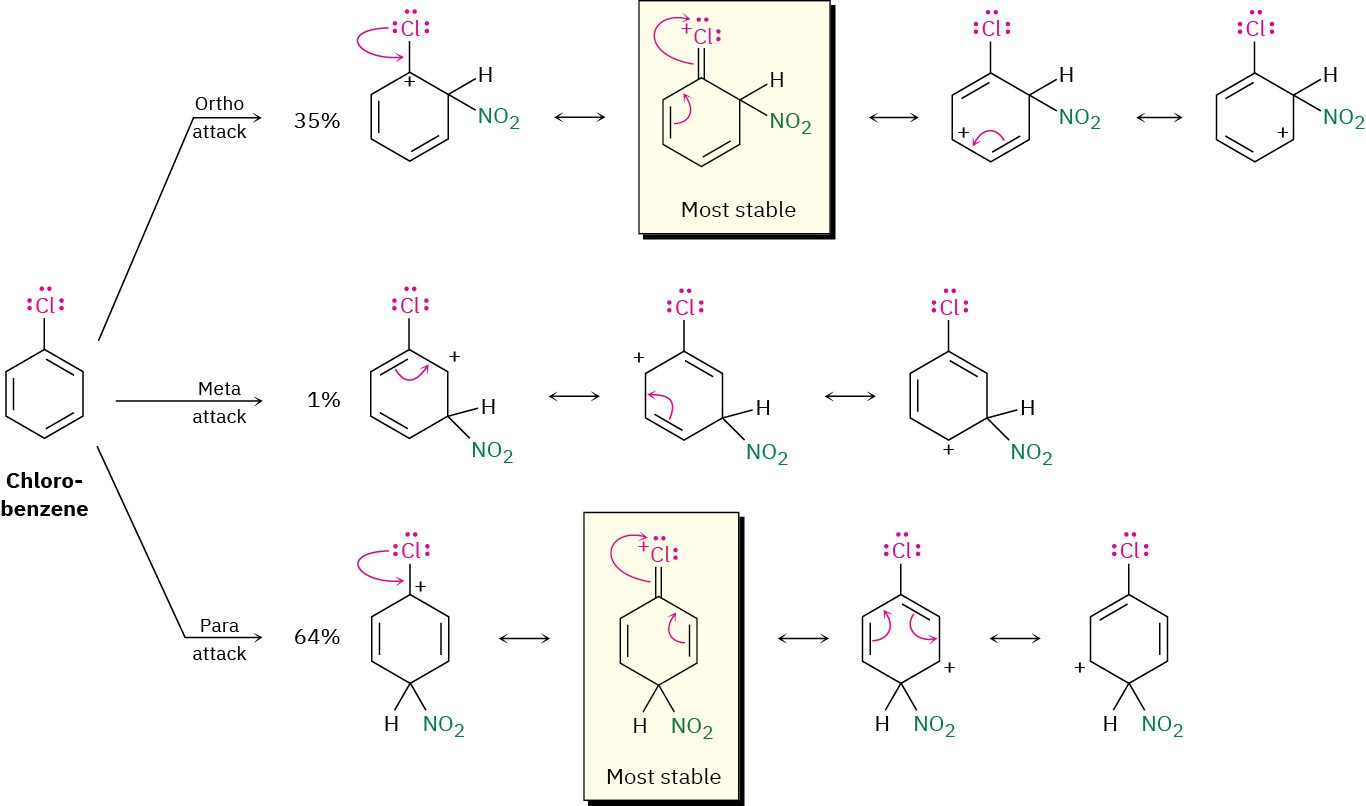
Figure 8.15 Carbocation intermediates in the nitration of chlorobenzene. The ortho and para intermediates are more stable than the meta intermediate because of electron donation of the halogen lone-pair electrons.
Note again that halogens, hydroxyl, alkoxyl, and amino groups all withdraw electrons inductively but donate electrons by resonance. Halogens have a stronger electron- withdrawing inductive effect but a weaker electron-donating resonance effect and are thus deactivators. Hydroxyl, alkoxyl, and amino groups have a weaker electron-withdrawing inductive effect but a stronger electron-donating resonance effect and are thus activators. All are ortho and para directors, however, because of the lone pair of electrons on the atom bonded to the aromatic ring.
Meta-Directing Deactivators
The influence of meta-directing substituents can be explained using the same kinds of arguments used for ortho and para directors. Look at the nitration of benzaldehyde, for instance (Figure 8.16). Of the three possible carbocation intermediates, the meta intermediate has three favorable resonance forms, whereas the ortho and para intermediates have only two. In both ortho and para intermediates, the third resonance form is unfavorable because it places the positive charge directly on the carbon that bears the aldehyde group, where it is disfavored by a repulsive interaction with the positively polarized carbon atom of the C=O group. Hence, the meta intermediate is more favored and is formed faster than the ortho and para intermediates.
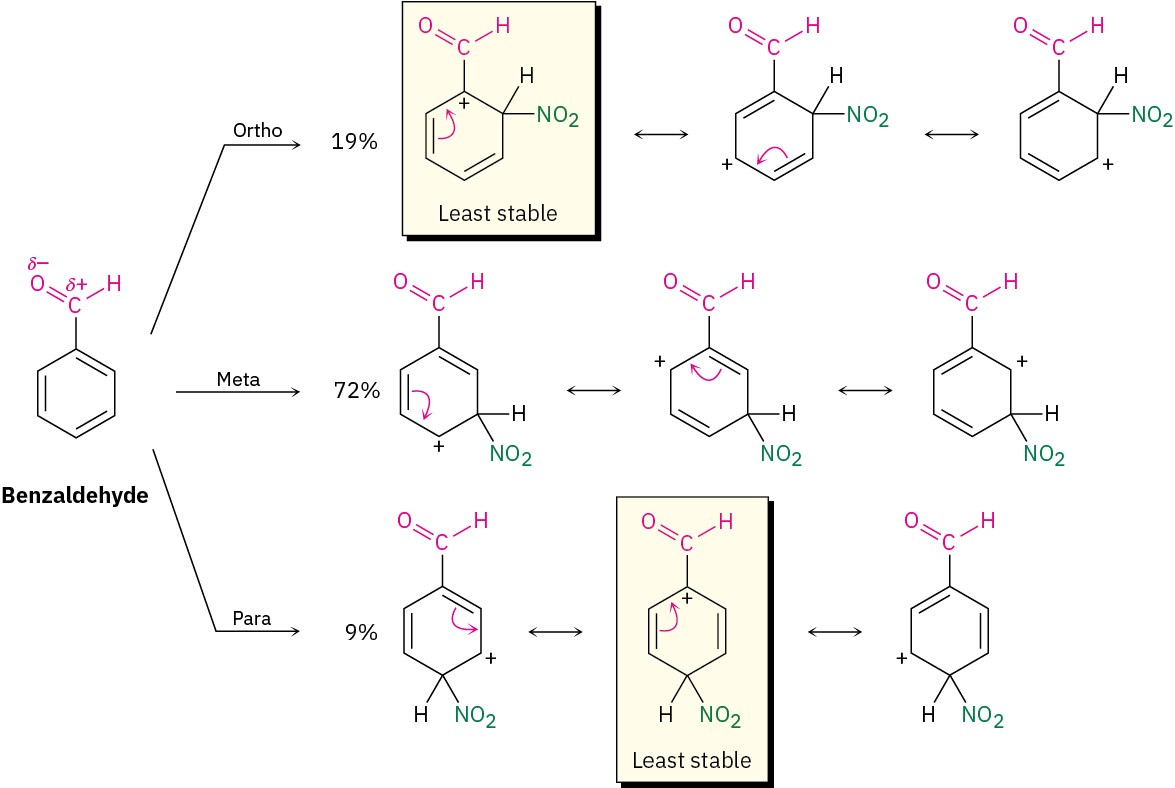 Figure 8.16 Carbocation intermediates in the nitration of benzaldehyde. The ortho and para intermediates are less stable than the meta intermediate. The meta intermediate is more favorable than ortho and para intermediates because it has three favorable resonance forms rather than two.
Figure 8.16 Carbocation intermediates in the nitration of benzaldehyde. The ortho and para intermediates are less stable than the meta intermediate. The meta intermediate is more favorable than ortho and para intermediates because it has three favorable resonance forms rather than two.
In general, any substituent that has a positively polarized atom (δ+) directly attached to the ring will make one of the resonance forms of the ortho and para intermediates unfavorable and will thus act as a meta director.
Problem 8.12
Draw resonance structures for the intermediates from the reaction of an electrophile at the ortho, meta, and para positions of nitrobenzene. Which intermediates are most stable?
A Summary of Substituent Effects in Electrophilic Aromatic Substitution
A summary of the activating and directing effects of substituents in electrophilic aromatic substitution is shown in Table 8.3.
Table 8.3 Substituent Effects in Electrophilic Aromatic Substitution
|
Reactivity |
Orienting effect |
Inductive effect |
Resonance effect |
|
|
–CH3 |
Activating |
Ortho, para |
Weak donating |
— |
|
–OH, –NH2 |
Activating |
Ortho, para |
Weak withdrawing |
Strong donating |
|
–F, –Cl |
Deactivating |
Ortho, para |
Strong withdrawing |
Weak donating |
|
–Br, –I |
||||
|
–NO2, –CN, |
Deactivating |
Meta |
Strong withdrawing |
Strong withdrawing |
|
–CHO, –CO2R |
||||
|
–COR, –CO2H |

