3.9 A Review of Isomerism
As noted on several previous occasions, isomers are compounds with the same chemical formula but different structures. We’ve seen several kinds of isomers in the past few chapters, and it’s a good idea at this point to see how they relate to one another (Figure 3.15).
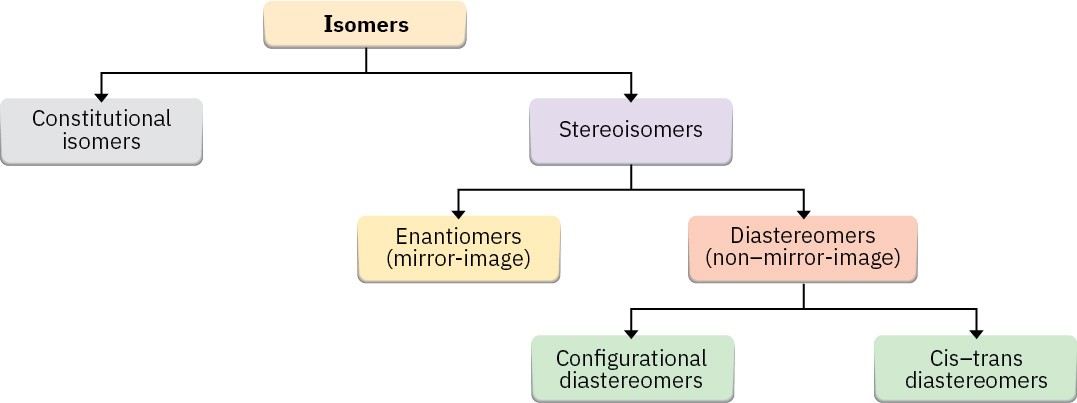 Figure 3.15 A summary of the different kinds of isomers.
Figure 3.15 A summary of the different kinds of isomers.
There are two fundamental types of isomers, both of which we’ve now encountered: constitutional isomers and stereoisomers.
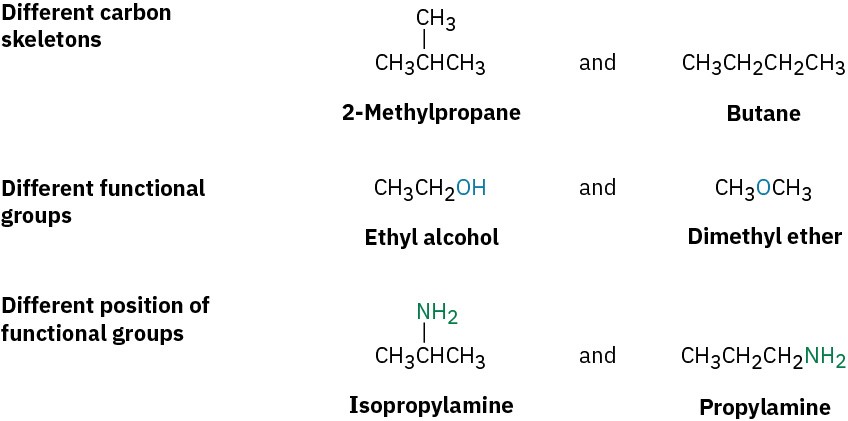
Constitutional isomers (Section 2.2) are compounds whose atoms are connected differently. Among the kinds of constitutional isomers we’ve seen are skeletal, functional, and positional isomers.
Stereoisomers (Section 2.9) are compounds whose atoms are connected in the same order but with a different spatial arrangement. Among the kinds of stereoisomers we’ve seen are enantiomers, diastereomers, and cis–trans isomers of cycloalkanes. Actually, cis–trans isomers are just a subclass of diastereomers because they are non–mirror-image stereoisomers: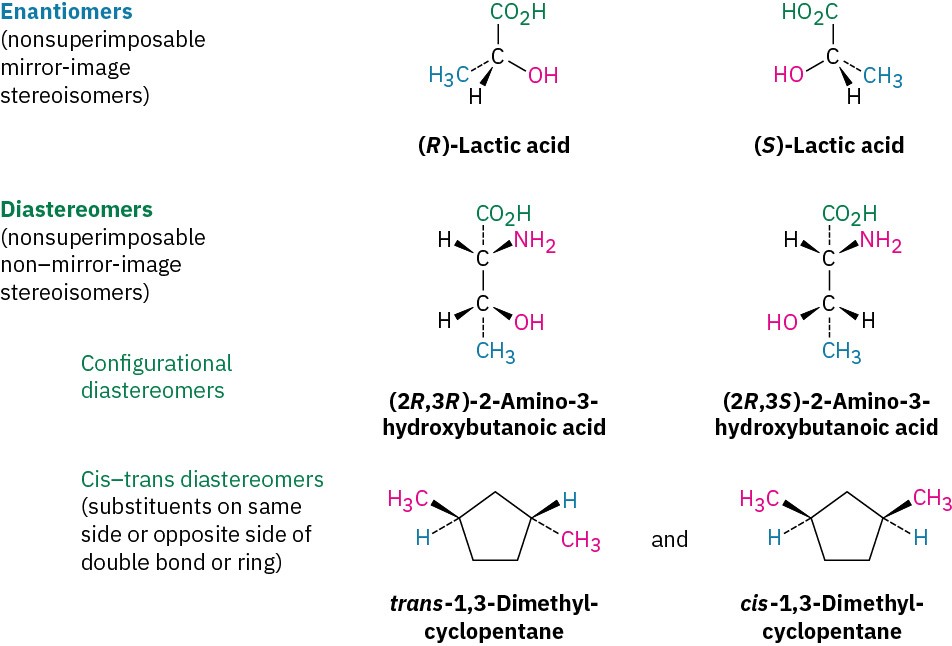 Problem 3.17
Problem 3.17
What kinds of isomers are the following pairs?
(a) (S)-5-Chloro-2-hexene and chlorocyclohexane
(b) (2R,3R)-Dibromopentane and (2S,3R)-dibromopentane

