7.11 A Summary of Reactivity: SN1, SN2, E1, E1cB, and E2
SN1, SN2, E1, E1cB, E2—how can you keep it all straight and predict what will happen in any given case? Will substitution or elimination occur? Will the reaction be bimolecular or unimolecular? There are no rigid answers to these questions, but it’s possible to recognize some trends and make some generalizations.
Primary alkyl halides SN2 substitution occurs if a good nucleophile is used, E2 elimination occurs if a strong, sterically hindered base is used, and E1cB elimination occurs if the leaving group is two carbons away from a carbonyl group.
Secondary alkyl halides SN2 substitution occurs if a weakly basic nucleophile is used in a polar aprotic solvent, E2 elimination predominates if a strong base is used, and E1cB elimination takes place if the leaving group is two carbons away from a carbonyl group.
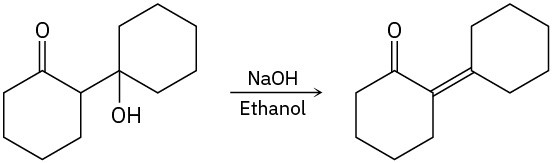
Tertiary alkyl halides E2 elimination occurs when a base is used, but SN1 substitution and E1 elimination occur together under neutral conditions, such as in pure ethanol or water. E1cB elimination takes place if the leaving group is two carbons away from a carbonyl group.
Worked Example 7.3: Predicting the Product and Mechanism of Reactions
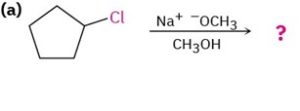
Tell whether each of the following reaction is likely to be SN1, SN2, E1, E1cB, or E2, and predict the product of each:
 Strategy:
Strategy:
Look carefully in each reaction at the structure of the substrate, the leaving group, the nucleophile, and the solvent. Then decide from the preceding summary which kind of reaction is likely to be favored.
Solution:
A secondary, substrate can undergo an SN2 reaction with a good nucleophile but will undergo an E2 reaction on treatment with a strong base. In this case, E2 reaction is likely to predominate.
![]() Problem 7.12
Problem 7.12
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, or E2:
(a)

(b)
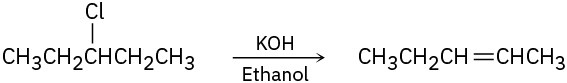
(c)
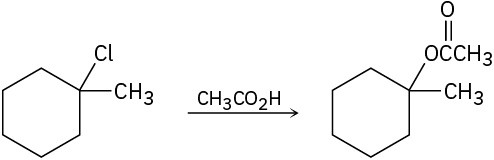
(d)