1.18 Acid and Base Strength
Different acids differ in their ability to donate H+. Stronger acids, such as HCl, react almost completely with water, whereas weaker acids, such as acetic acid (CH3CO2H), react only slightly. The exact strength of a given acid HA in water solution is described using the acidity constant (Ka) for the acid-dissociation equilibrium. Recall from general chemistry that the concentration of solvent is ignored in the equilibrium expression and that brackets [ ] around a substance refer to the concentration of the enclosed species in moles per liter.
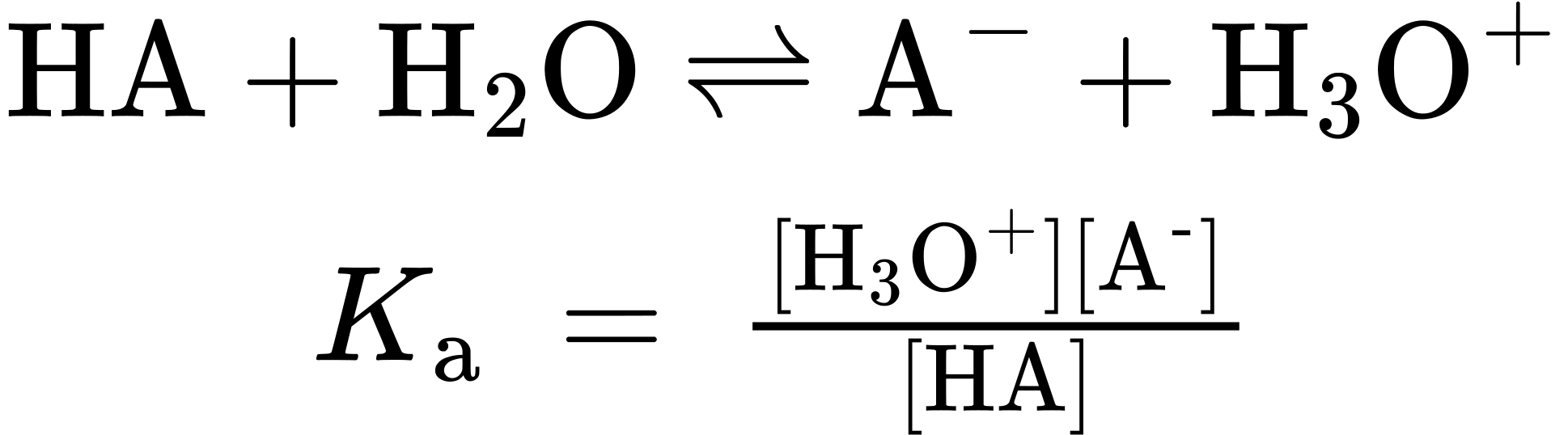 Stronger acids have their equilibria toward the right and thus have larger acidity constants, whereas weaker acids have their equilibria toward the left and have smaller acidity constants. The range of Ka values for different acids is enormous, running from about 1015 for the strongest acids to about 10–60 for the weakest. Common inorganic acids such as H2SO4, HNO3, and HCl have Ka’s in the range of 102 to 109, while organic acids generally have Ka’s in the range of 10–5 to 10–15. As you gain experience, you’ll develop a rough feeling for which acids are “strong” and which are “weak” (always remembering that the terms are relative, not absolute).
Stronger acids have their equilibria toward the right and thus have larger acidity constants, whereas weaker acids have their equilibria toward the left and have smaller acidity constants. The range of Ka values for different acids is enormous, running from about 1015 for the strongest acids to about 10–60 for the weakest. Common inorganic acids such as H2SO4, HNO3, and HCl have Ka’s in the range of 102 to 109, while organic acids generally have Ka’s in the range of 10–5 to 10–15. As you gain experience, you’ll develop a rough feeling for which acids are “strong” and which are “weak” (always remembering that the terms are relative, not absolute).
Acid strengths are normally expressed using pKa values rather than Ka values, where the pKa is the negative common logarithm of the Ka:
p𝐾a = −log 𝐾a
A stronger acid (larger Ka) has a smaller pKa, and a weaker acid (smaller Ka) has a larger pKa. Table 1.6 lists the pKa’s of some common acids in order of their strength, and a more comprehensive table is given in Appendix B.
Notice that the pKa value shown in Table 1.6 for water is 15.74, which results from the following calculation. Because water is both the acid and the solvent, the equilibrium expression is
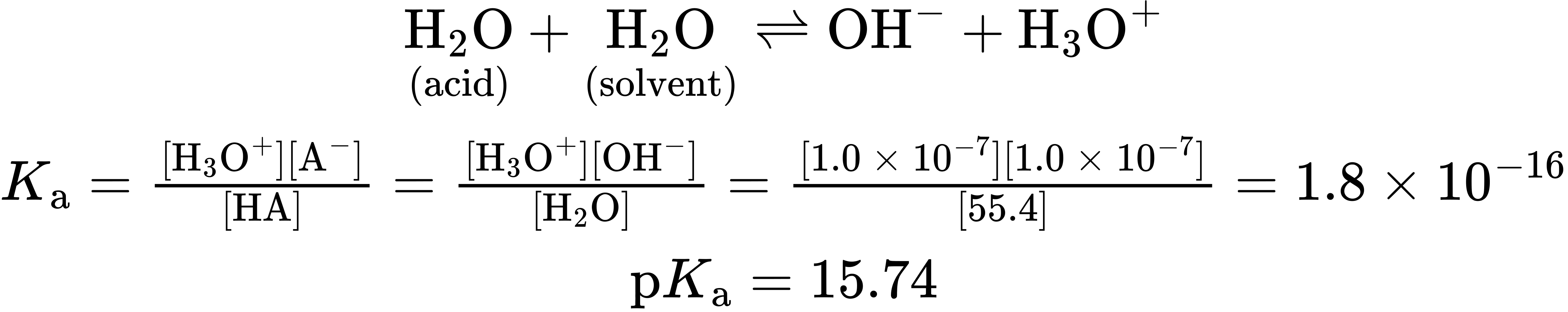
The numerator in this expression is the so-called ion-product constant for water, Kw = [H3O+][OH–] = 1.00 × 10–14, and the denominator is the molar concentration of pure water, [H2O] = 55.4 M at 25 °C. The calculation is artificial in that the concentration of “solvent” water is ignored while the concentration of “acid” water is not, but it is nevertheless useful for making a comparison of water with other weak acids on a similar footing.
Notice also in Table 1.6 that there is an inverse relationship between the acid strength of an acid and the base strength of its conjugate base. A strong acid has a weak conjugate base, and a weak acid has a strong conjugate base. To understand this inverse relationship, think about what is happening to the acidic hydrogen in an acid–base reaction. A strong acid is one that loses H+ easily, meaning that its conjugate base holds the H+ weakly and is therefore a weak base. A weak acid is one that loses H+ with difficulty, meaning that its conjugate base holds the proton tightly and is therefore a strong base. The fact that HCl is a strong acid, for example, means that Cl– does not hold H+ tightly and is thus a weak base.
Water, on the other hand, is a weak acid, meaning that OH– holds H+ tightly and is a strong base.
Table 1.6 Relative Strengths of Some Common Acids and Their Conjugate Bases
|
|
Name |
pKa |
Conjugate base |
Name |
|
|
|
|
CH3CH2OH |
Ethanol |
16.00 |
CH3CH2O– |
Ethoxide ion |
|
|
H2O |
Water |
15.74 |
HO– |
Hydroxide ion |
||
|
HCN |
Hydrocyanic acid |
9.31 |
CN– |
Cyanide ion |
||
|
H2PO4– |
Dihydrogen phosphate ion |
7.21 |
HPO42– |
Hydrogen phosphate ion |
||
|
CH3CO2H |
Acetic acid |
4.76 |
CH3CO2– |
Acetate ion |
||
|
H3PO4 |
Phosphoric acid |
2.16 |
H2PO4– |
Dihydrogen phosphate ion |
||
|
HNO3 |
Nitric acid |
–1.3 |
NO3– |
Nitrate ion |
||
|
HCl |
Hydrochloric acid |
–7.0 |
Cl– |
Chloride ion |
Problem 1.25
 Problem 1.26
Problem 1.26
Amide ion, H2N–, is a much stronger base than hydroxide ion, HO–. Which is the stronger acid, NH3 or H2O? Explain.



