9.6 Alcohols from Carbonyl Compounds: Grignard Reaction
Grignard reagents (RMgX), prepared by reaction of organohalides with magnesium (Section 6.4), react with carbonyl compounds to yield alcohols in much the same way that hydride reducing agents do. Just as carbonyl reduction involves addition of a hydride ion nucleophile to the C=O bond, Grignard reaction involves addition of a carbanion nucleophile (R:−+MgX).
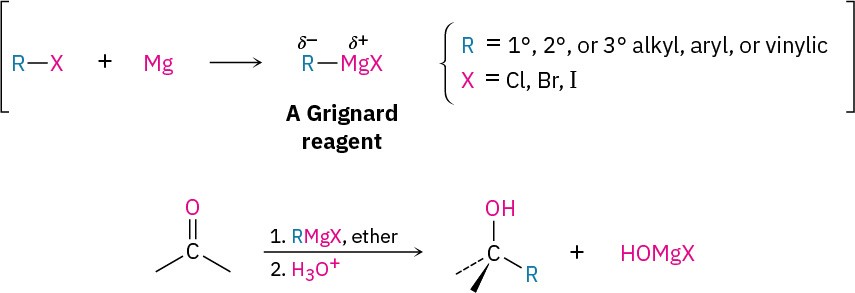 The nucleophilic addition reaction of Grignard reagents to carbonyl compounds has no direct counterpart in biological chemistry because organomagnesium compounds are too strongly basic to exist in an aqueous medium. Nevertheless, the reaction is worth understanding for two reasons. First, the reaction is an unusually broad and useful method of alcohol synthesis and demonstrates again the relative freedom with which chemists can operate in the laboratory. Second, the reaction does have an indirect biological counterpart, for we’ll see later that the addition of stabilized carbon nucleophiles to carbonyl compounds is used in almost all metabolic pathways as the major process for forming carbon–carbon bonds.
The nucleophilic addition reaction of Grignard reagents to carbonyl compounds has no direct counterpart in biological chemistry because organomagnesium compounds are too strongly basic to exist in an aqueous medium. Nevertheless, the reaction is worth understanding for two reasons. First, the reaction is an unusually broad and useful method of alcohol synthesis and demonstrates again the relative freedom with which chemists can operate in the laboratory. Second, the reaction does have an indirect biological counterpart, for we’ll see later that the addition of stabilized carbon nucleophiles to carbonyl compounds is used in almost all metabolic pathways as the major process for forming carbon–carbon bonds.
As examples of their addition to carbonyl compounds, Grignard reagents react with formaldehyde, H2C=O, to give primary alcohols, with aldehydes to give secondary alcohols, and with ketones to give tertiary alcohols.
Esters react with Grignard reagents to yield tertiary alcohols in which two of the substituents bonded to the hydroxyl-bearing carbon have come from the Grignard reagent, just as LiAlH4 reduction of an ester adds two hydrogens.
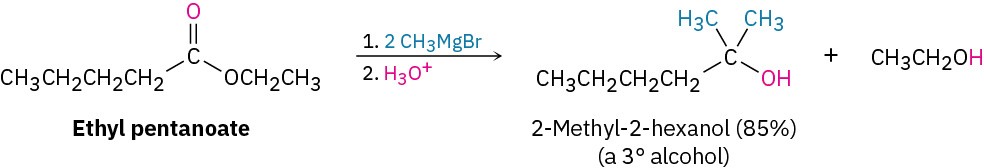
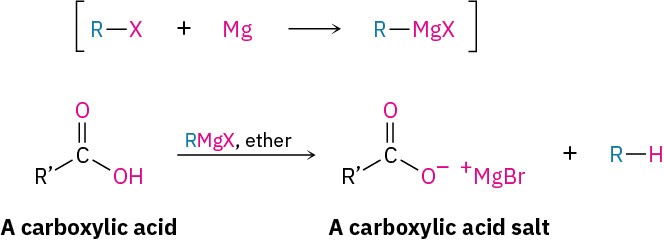
Carboxylic acids don’t give addition products when treated directly with Grignard reagents because the acidic carboxyl hydrogen reacts with the basic Grignard reagent to yield a hydrocarbon and the magnesium salt of the acid.
 The Grignard reaction, although useful, does have limitations. One major problem is that a Grignard reagent can’t be prepared from an organohalide if other reactive functional groups are present in the same molecule. For example, a compound that is both an alkyl halide and a ketone can’t form a Grignard reagent because it would react with itself.
The Grignard reaction, although useful, does have limitations. One major problem is that a Grignard reagent can’t be prepared from an organohalide if other reactive functional groups are present in the same molecule. For example, a compound that is both an alkyl halide and a ketone can’t form a Grignard reagent because it would react with itself.
Similarly, a compound that is both an alkyl halide and a carboxylic acid, alcohol, or amine can’t form a Grignard reagent because the acidic RCO2H, ROH, or RNH2 hydrogen present in the same molecule would react with the basic Grignard reagent as rapidly as it forms. In general, Grignard reagents can’t be prepared from alkyl halides that contain the following functional groups (FG):
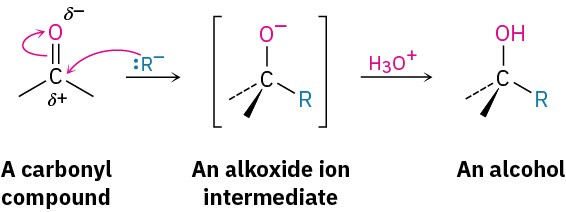
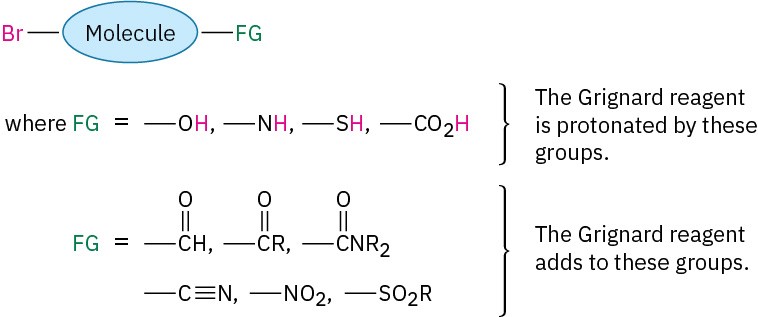 As with the reduction of carbonyl compounds discussed in the previous section, we’ll defer a detailed treatment of the Grignard reactions until Chapter 10. For the moment, it’s sufficient to note that Grignard reagents act as nucleophilic carbanions (:R–) and that their addition to a carbonyl compound is analogous to the addition of hydride ion. The intermediate is an alkoxide ion, which is protonated by addition of H3O+ in a second step.
As with the reduction of carbonyl compounds discussed in the previous section, we’ll defer a detailed treatment of the Grignard reactions until Chapter 10. For the moment, it’s sufficient to note that Grignard reagents act as nucleophilic carbanions (:R–) and that their addition to a carbonyl compound is analogous to the addition of hydride ion. The intermediate is an alkoxide ion, which is protonated by addition of H3O+ in a second step.
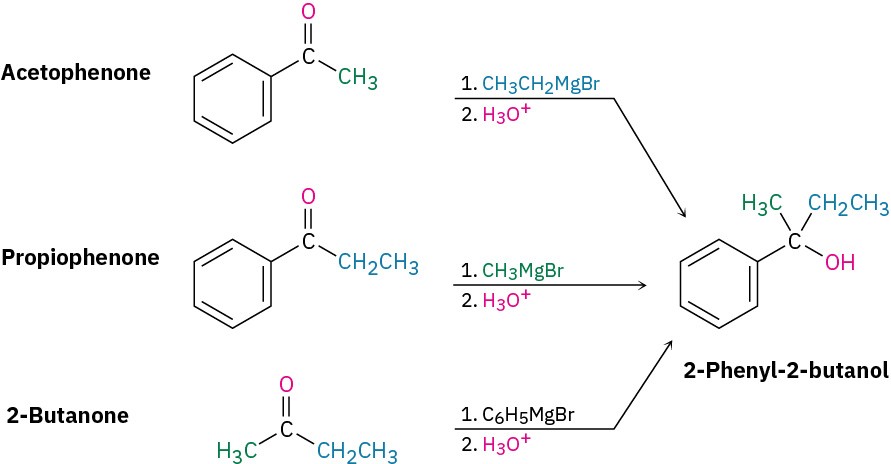
Worked Example 9.3: Using a Grignard Reaction to Synthesize an Alcohol
How could you use the addition of a Grignard reagent to a ketone to synthesize 2-phenyl-2- butanol?
Strategy
Draw the product, and identify the three groups bonded to the alcohol carbon atom. One of the three will have come from the Grignard reagent, and the remaining two will have come from the ketone.
Solution
2-Phenyl-2-butanol has a methyl group, an ethyl group, and a phenyl group (–C6H5) attached to the alcohol carbon atom. Thus, the possibilities are addition of ethylmagnesium bromide to acetophenone, addition of methylmagnesium bromide to propiophenone, and addition of phenylmagnesium bromide to 2-butanone.
Worked Example 9.4: Using a Grignard Reaction to Synthesize an Alcohol
How could you use the reaction of a Grignard reagent with a carbonyl compound to synthesize 2-methyl-2-pentanol?
Strategy
Draw the product, and identify the three groups bonded to the alcohol carbon atom. If the three groups are all different, the starting carbonyl compound must be a ketone. If two of the three groups are identical, the starting carbonyl compound could be either a ketone or an ester.
Solution
In the present instance, the product is a tertiary alcohol with two methyl groups and one propyl group. Starting from a ketone, the possibilities are addition of methylmagnesium bromide to 2-pentanone and addition of propylmagnesium bromide to acetone.
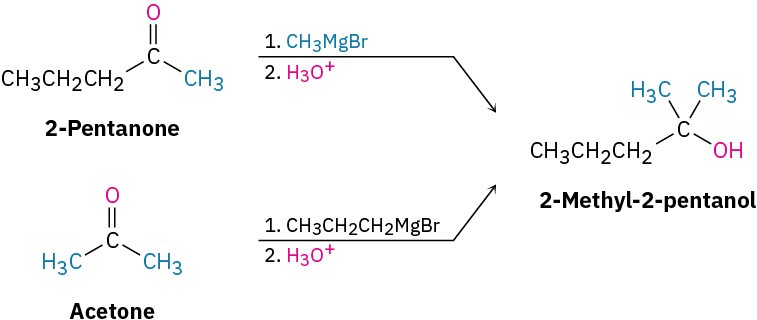
Starting from an ester, the only possibility is addition of methylmagnesium bromide to an ester of butanoic acid, such as methyl butanoate.
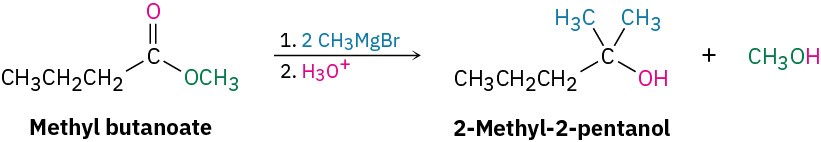 Problem 9.8
Problem 9.8
Show the products obtained from addition of methylmagnesium bromide to the following compounds:
(a) Cyclopentanone
(b) Benzophenone (diphenyl ketone)
(c) 3-Hexanone
Problem 9.9
Use a Grignard reaction to prepare the following alcohols:
(a) 2-Methyl-2-propanol
(b) 1-Methylcyclohexanol
(c) 3-Methyl-3-pentanol
(d) 2-Phenyl-2-butanol
(e) Benzyl alcohol
(f) 4-Methyl-1-pentanol
Problem 9.10
Use the reaction of a Grignard reagent with a carbonyl compound to synthesize the following compound: