9.5 Alcohols from Carbonyl Compounds: Reduction
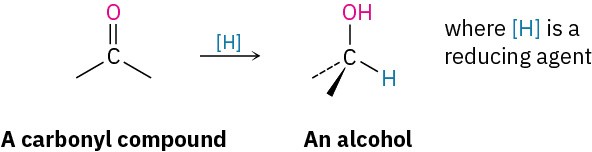
The most general method for preparing alcohols, both in the laboratory and in living organisms, is by reduction of a carbonyl compound. Just as reduction of an alkene adds hydrogen to a C=C bond to give an alkane (Section 5.5), reduction of a carbonyl compound adds hydrogen to a C=O bond to give an alcohol. All kinds of carbonyl compounds can be reduced, including aldehydes, ketones, carboxylic acids, and esters.
Reduction of Aldehydes and Ketones
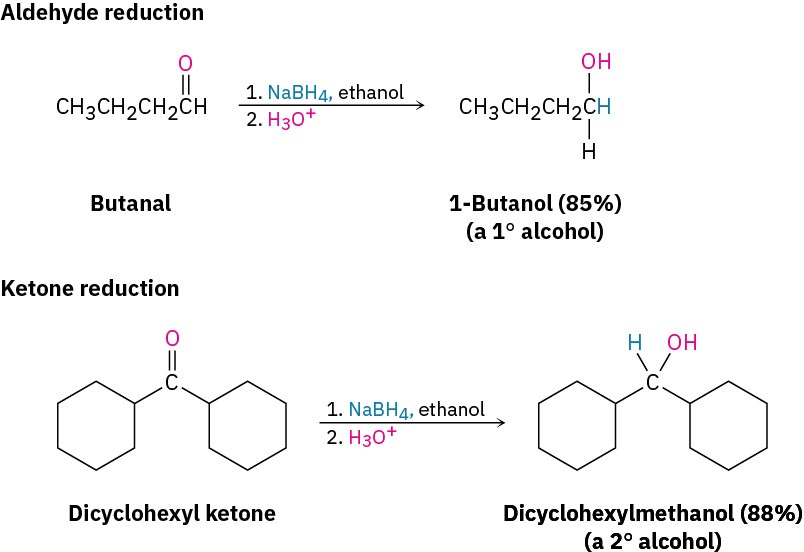
Aldehydes are easily reduced to give primary alcohols, and ketones are reduced to give secondary alcohols.

Dozens of reagents are used in the laboratory to reduce aldehydes and ketones, depending on the circumstances, but sodium borohydride, NaBH4, is usually chosen because of its safety and ease of handling. Sodium borohydride is a white, crystalline solid that can be weighed in the open atmosphere and used in either water or alcohol solution.
 Lithium aluminum hydride, LiAlH4, is another reducing agent often used for reduction of aldehydes and ketones. A grayish powder that is soluble in ether and tetrahydrofuran, LiAlH4 is much more reactive than NaBH4 but also much more dangerous. It reacts violently with water and decomposes explosively when heated above 120 °C.
Lithium aluminum hydride, LiAlH4, is another reducing agent often used for reduction of aldehydes and ketones. A grayish powder that is soluble in ether and tetrahydrofuran, LiAlH4 is much more reactive than NaBH4 but also much more dangerous. It reacts violently with water and decomposes explosively when heated above 120 °C.
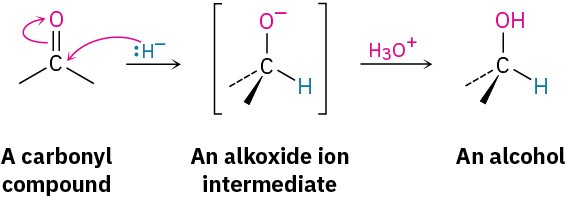
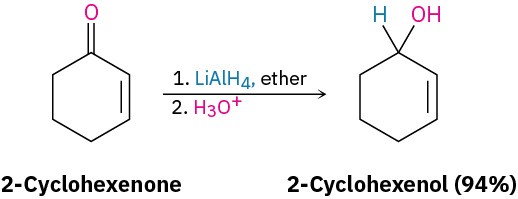 We’ll defer a detailed discussion of these reductions until Chapter 10. For the moment, we’ll simply note that they involve the addition of a nucleophilic hydride ion (:H–) to the positively polarized, electrophilic carbon atom of the carbonyl group. The initial product is an alkoxide ion, which is protonated by addition of H3O+ in a second step to yield the alcohol product.
We’ll defer a detailed discussion of these reductions until Chapter 10. For the moment, we’ll simply note that they involve the addition of a nucleophilic hydride ion (:H–) to the positively polarized, electrophilic carbon atom of the carbonyl group. The initial product is an alkoxide ion, which is protonated by addition of H3O+ in a second step to yield the alcohol product.
Reduction of Carboxylic Acids and Esters
Carboxylic acids and esters are reduced to give primary alcohols.
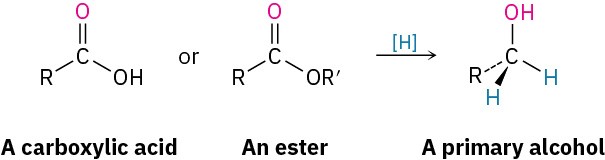 These reactions aren’t as rapid as the reductions of aldehydes and ketones. NaBH4 reduces esters very slowly and does not reduce carboxylic acids at all. Instead, carboxylic acid and ester reductions are usually carried out with the more reactive reducing agent LiAlH4. All carbonyl groups, including acids, esters, ketones, and aldehydes, are reduced by LiAlH4.
These reactions aren’t as rapid as the reductions of aldehydes and ketones. NaBH4 reduces esters very slowly and does not reduce carboxylic acids at all. Instead, carboxylic acid and ester reductions are usually carried out with the more reactive reducing agent LiAlH4. All carbonyl groups, including acids, esters, ketones, and aldehydes, are reduced by LiAlH4.
Note that one hydrogen atom is delivered to the carbonyl carbon atom during aldehyde and ketone reductions but that two hydrogens become bonded to the former carbonyl carbon during carboxylic acid and ester reductions. 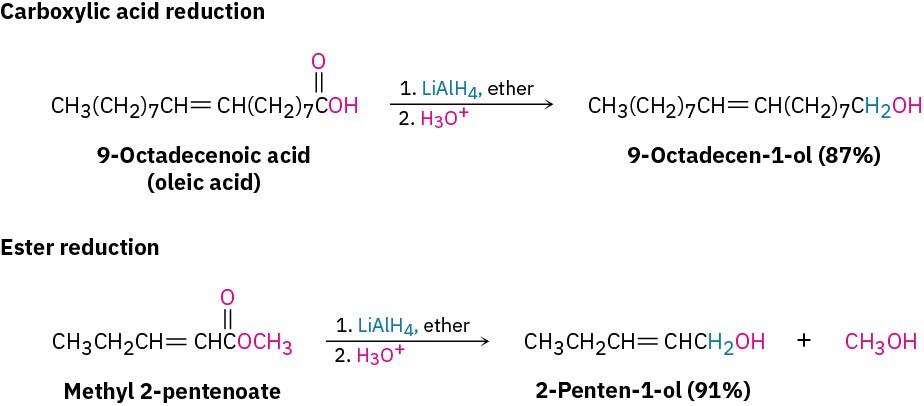 Worked Example 9.2: Identifying a Reactant, Given the Product
Worked Example 9.2: Identifying a Reactant, Given the Product
What carbonyl compounds would you reduce to obtain the following alcohols?

Strategy
Identify the target alcohol as primary, secondary, or tertiary. A primary alcohol can be prepared by reduction of an aldehyde, an ester, or a carboxylic acid; a secondary alcohol can be prepared by reduction of a ketone; and a tertiary alcohol can’t be prepared by reduction.
Solution
(a) The target molecule is a secondary alcohol, which can be prepared only by reduction of a ketone. Either NaBH4 or LiAlH4 can be used.
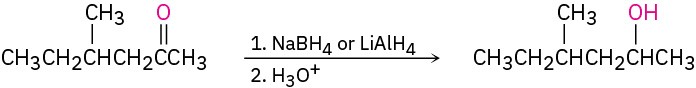 (b) The target molecule is a primary alcohol, which can be prepared by reduction of an aldehyde, an ester, or a carboxylic acid. LiAlH4 is needed for the ester and carboxylic acid reductions.
(b) The target molecule is a primary alcohol, which can be prepared by reduction of an aldehyde, an ester, or a carboxylic acid. LiAlH4 is needed for the ester and carboxylic acid reductions.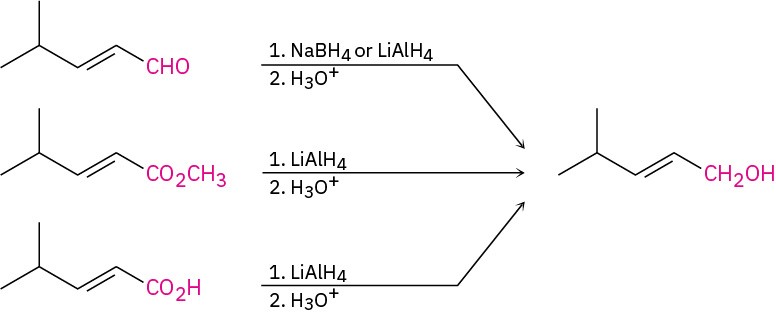 Problem 9.6
Problem 9.6
What reagent would you use to accomplish each of the following reactions?
(a)

(b)

(c)
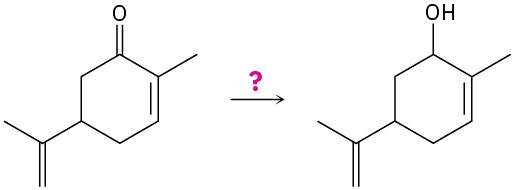
Problem 9.7
What carbonyl compounds give the following alcohols on reduction with LiAlH4? Show all possibilities.
(a)
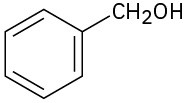
(b)
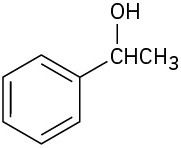
(c)
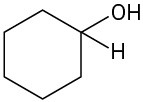
(d)