8.5 Alkylation and Acylation of Aromatic Rings: The Friedel–Crafts Reaction
Among the most useful electrophilic aromatic substitution reactions in the laboratory is alkylation—the introduction of an alkyl group onto the benzene ring. Called the Friedel– Crafts reaction after its founders in 1877, Charles Friedel and James Crafts, the reaction is carried out by treating an aromatic compound with an alkyl chloride, RCl, in the presence of AlCl3 to generate a carbocation electrophile, R+. Aluminum chloride catalyzes the reaction by helping the alkyl halide to generate a carbocation in much the same way that FeBr3 catalyzes aromatic brominations by polarizing Br2 (Section 8.3). Loss of H+ then completes the reaction (Figure 8.9).
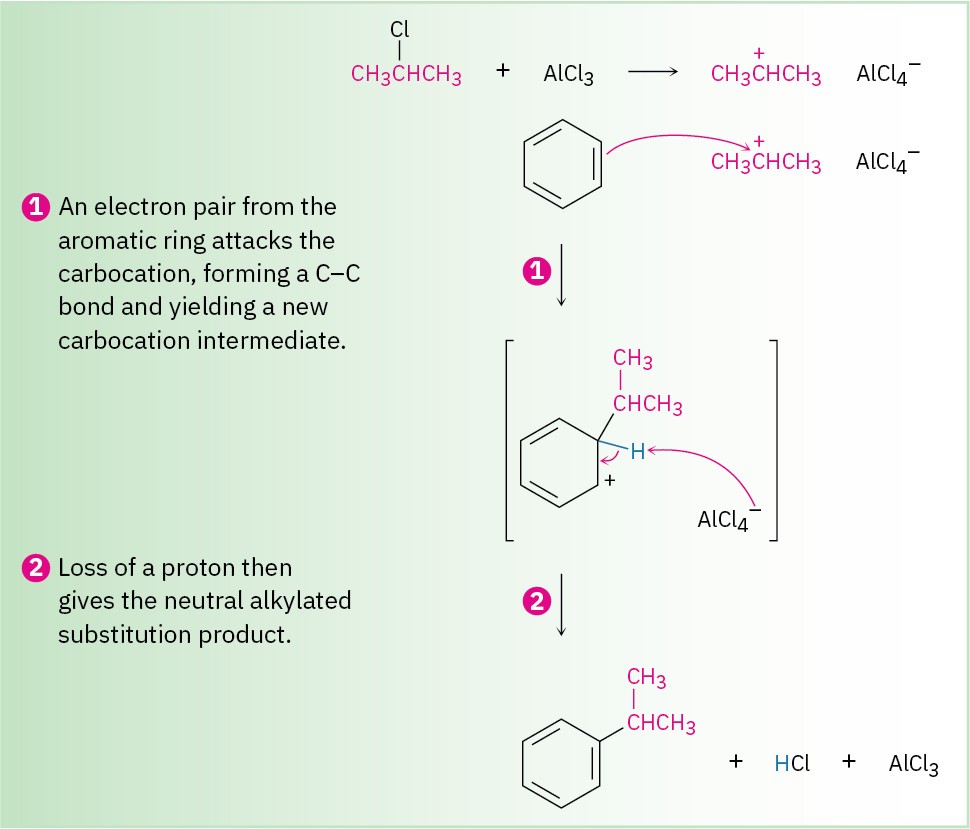
Figure 8.9 MECHANISM: Mechanism for the Friedel–Crafts alkylation reaction of benzene with 2-chloropropane to yield isopropylbenzene (cumene). The electrophile is a carbocation, generated by AlCl3-assisted dissociation of an alkyl halide.
Despite its utility, the Friedel–Crafts alkylation has several limitations. For one thing, only alkyl halides can be used. Aromatic (aryl) halides and vinylic halides don’t react because aryl and vinylic carbocations are too high in energy to form under Friedel–Crafts conditions.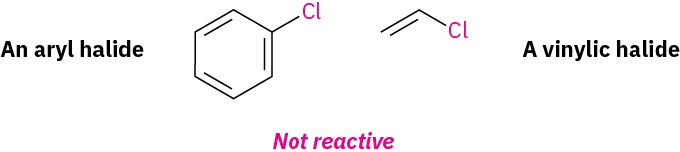
Another limitation is that Friedel–Crafts reactions don’t succeed on aromatic rings that are substituted either by a strongly electron-withdrawing group such as carbonyl (C=O) or by a basic amino group that can be protonated. We’ll see in the next section that the presence of a substituent group already on a ring can have a dramatic effect on that ring’s reactivity to further electrophilic substitution. Rings that contain any of the substituents listed in Figure 8.10 do not undergo Friedel–Crafts alkylation.
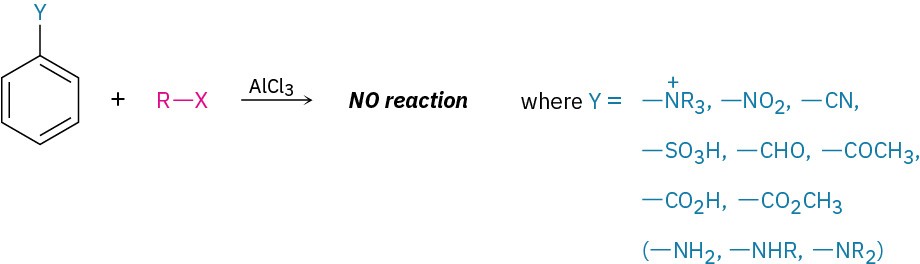 Figure 8.10 Limitations on the aromatic substrate in Friedel–Crafts reactions. No reaction occurs if the substrate has either an electron-withdrawing substituent or a basic amino group.
Figure 8.10 Limitations on the aromatic substrate in Friedel–Crafts reactions. No reaction occurs if the substrate has either an electron-withdrawing substituent or a basic amino group.
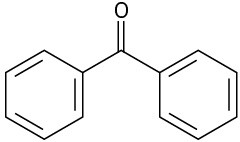
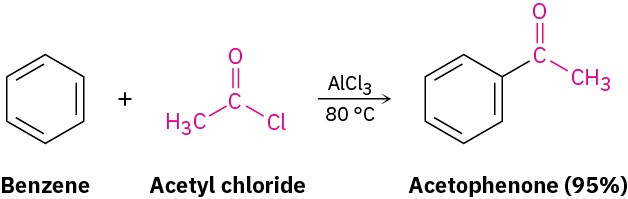
Just as an aromatic ring is alkylated by reaction with an alkyl chloride, it is acylated by reaction with a carboxylic acid chloride, RCOCl, in the presence of AlCl3. That is, an acyl group (–COR; pronounced a-sil) is substituted onto the aromatic ring. For example, reaction of benzene with acetyl chloride yields the ketone acetophenone.
 Problem 8.7
Problem 8.7
Identify the carboxylic acid chloride that might be used in a Friedel–Crafts acylation reaction to prepare each of the following acylbenzenes:
(a)
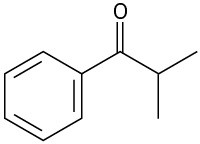
(b)