Additional Problems 1
Visualizing Chemistry
Problem 1.30
Convert each of the following molecular models into a skeletal structure, and give the formula of 1-18 each. Only the connections between atoms are shown; multiple bonds are not indicated (black = C, red = O, blue = N, gray = H).
(a)
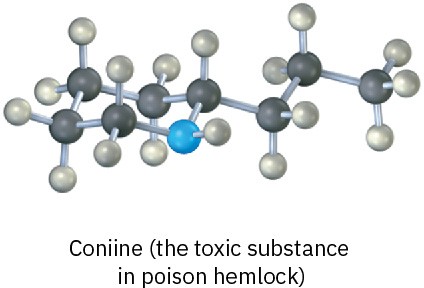
(b)
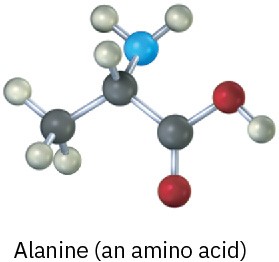
Problem 1.31
The following model is a representation of citric acid, the key substance in the so-called citric acid cycle, by which food molecules are metabolized in the body. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure by indicating the positions of multiple bonds and lone-pair electrons (black = C, red = O, gray = H).
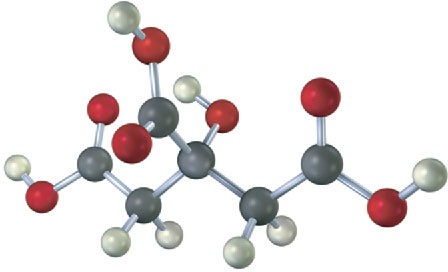
Problem 1.32
The following model is a representation of acetaminophen, a pain reliever sold in drugstores under a variety of names, including Tylenol. Identify the hybridization of each carbon atom in acetaminophen, and tell which atoms have lone pairs of electrons (black = C, red = O, blue = N, gray = H).
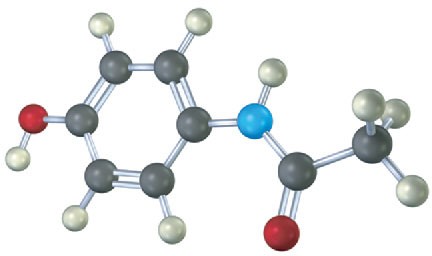
Problem 1.33
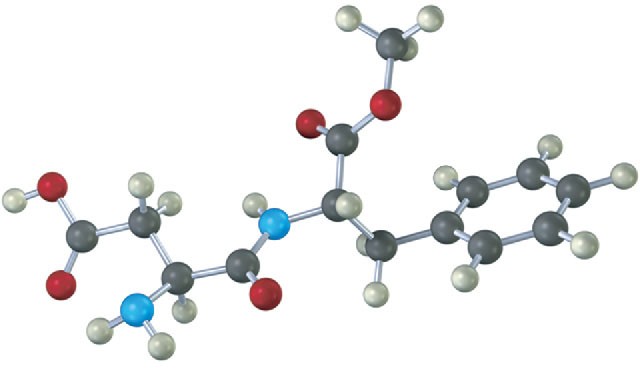
The following model is a representation of aspartame, C14H18N2O5, known commercially under many names, including NutraSweet. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure for aspartame, and indicate the positions of multiple bonds (black = C, red = O, blue = N, gray = H).
Electron Configurations
Problem 1.34
How many valence electrons does each of the following dietary trace elements have?
(a) Zinc (b) Iodine (c) Silicon (d) Iron
Problem 1.35
Give the ground-state electron configuration for each of the following elements:
(a) Potassium (b) Arsenic (c) Aluminum (d) Germanium
Electron-Dot and Line-Bond Structures
Problem 1.36
What are likely formulas for the following molecules?
(a) NH?OH
(b) AlCl?
(c) CF2Cl?
(d) CH?O
Problem 1.37
Why can’t molecules with the following formulas exist?
(a) CH5
(b) C2H6N
(c) C3H5Br2
Problem 1.38
Draw an electron-dot structure for acetonitrile, C2H3N, which contains a carbon–nitrogen triple bond. How many electrons does the nitrogen atom have in its outer shell? How many are bonding, and how many are nonbonding?
Problem 1.39
Draw a line-bond structure for vinyl chloride, C2H3Cl, the starting material from which PVC poly(vinyl chloride) plastic is made.
Problem 1.40
Fill in any nonbonding valence electrons that are missing from the following structures:
(a)
(b)
(c)
Problem 1.41
Convert the following line-bond structures into molecular formulas:
(a)
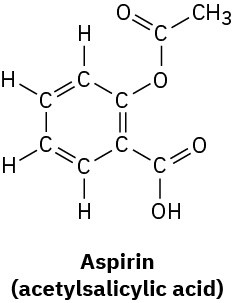
(b)
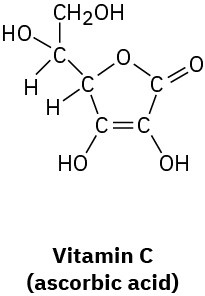
(c)
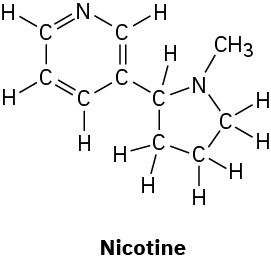
(d)
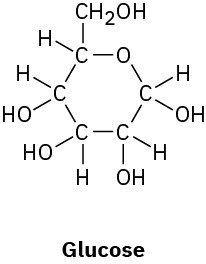
Problem 1.42
Convert the following molecular formulas into line-bond structures that are consistent with valence rules:
(a) C3H8
(b) CH5N
(c) C2H6O (2 possibilities)
(d) C3H7Br (2 possibilities)
(e) C2H4O (3 possibilities)
(f) C3H9N (4 possibilities)
Problem 1.43
Draw a three-dimensional representation of the oxygen-bearing carbon atom in ethanol, CH3CH2OH, using the standard convention of solid, wedged, and dashed lines.
Problem 1.44
Oxaloacetic acid, an important intermediate in food metabolism, has the formula C4H4O5 and contains three C=O bonds and two O–H bonds. Propose two possible structures.
Problem 1.45
Draw structures for the following molecules, showing lone pairs:
(a) Acrylonitrile, C3H3N, which contains a carbon–carbon double bond and a carbon–nitrogen triple bond
(b) Ethyl methyl ether, C3H8O, which contains an oxygen atom bonded to two carbons
(c) Butane, C4H10, which contains a chain of four carbon atoms
(d) Cyclohexene, C6H10, which contains a ring of six carbon atoms and one carbon–carbon double bond
Hybridization
Problem 1.46
What is the hybridization of each carbon atom in acetonitrile?
Problem 1.47
What kind of hybridization do you expect for each carbon atom in the following molecules?
(a)
![]()
(b)
![]()
(c)
![]()
(d)
![]()
Problem 1.48
What is the shape of benzene, and what hybridization do you expect for each carbon?
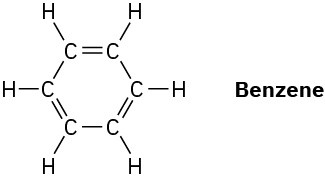
Problem 1.49
What bond angle do you expect for each of the indicated atoms, and what kind of hybridization do you expect for the central atom in each molecule?
(a)
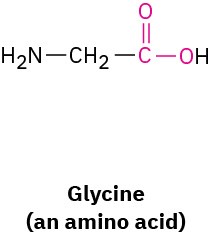
(b)
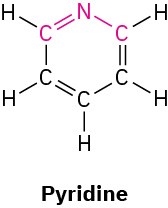
(c)
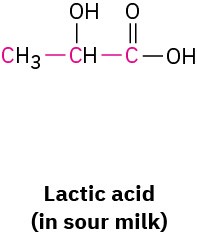
Problem 1.50
Propose structures for molecules that meet the following descriptions:
(a) Contains two sp2-hybridized carbons and two sp3-hybridized carbons
(b) Contains only four carbons, all of which are sp2-hybridized
(c) Contains two sp-hybridized carbons and two sp2-hybridized carbons
Problem 1.51
What kind of hybridization do you expect for each carbon atom in the following molecules:
(a)
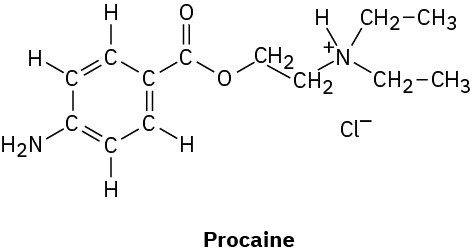
(b)
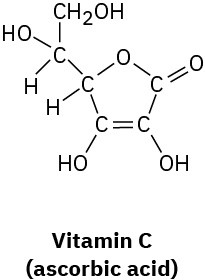
Problem 1.52
Pyridoxal phosphate, a close relative of vitamin B6, is involved in a large number of metabolic reactions. What is the hybridization and the bond angle for each nonterminal atom?
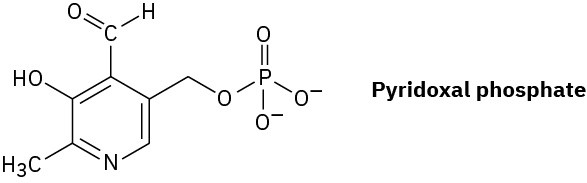
Skeletal Structures
Problem 1.53
Convert the following structures into skeletal drawings:
(a)
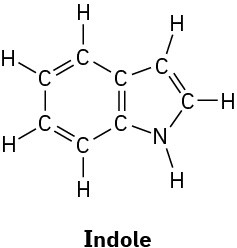
(b)
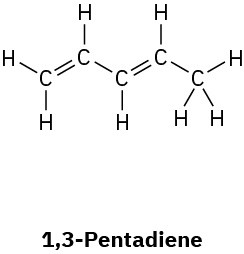
(c)
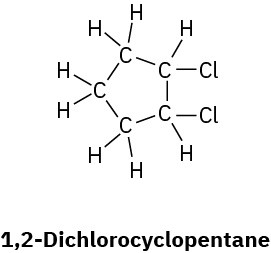
(d)
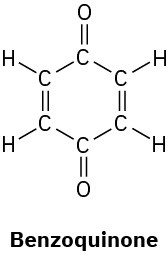
Problem 1.54
How many hydrogens are bonded to each carbon atom in the following substances, and what is the molecular formula of each?
(a)
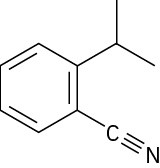
(b)
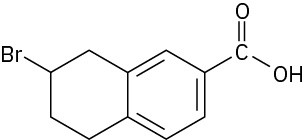
(c)
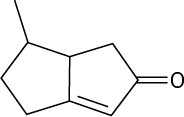
Problem 1.55
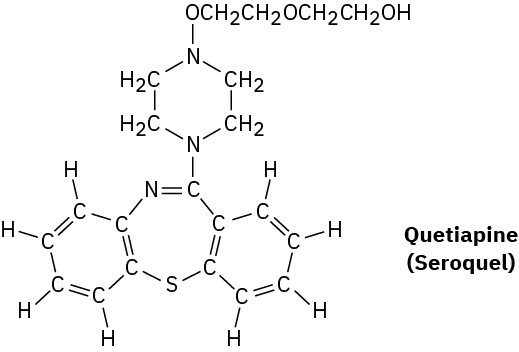
Quetiapine, marketed as Seroquel, is a heavily prescribed antipsychotic drug used in the treatment of schizophrenia and bipolar disorder. Convert the following representation into a skeletal structure, and give the molecular formula of quetiapine.
Problem 1.56
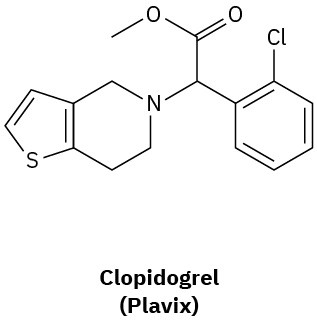
How many hydrogens are bonded to each carbon atom in (a) the antiinfluenza agent oseltamivir, marketed as Tamiflu, and (b) the platelet aggregation inhibitor clopidogrel, marketed as Plavix? Give the molecular formula of each.
(a)
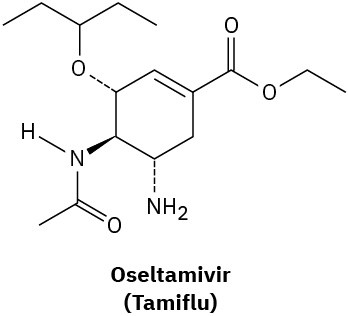
(b)
Mechanism Problems
Problem 1.57
Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds.
(a)
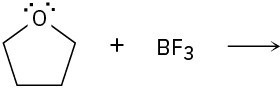
(b)
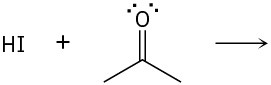
(c)
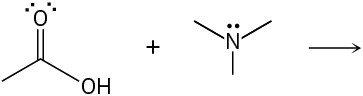
Problem 1.58
Use curved arrows to draw the protonated form of the following Lewis bases upon reacting with H+.
(a)
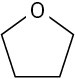
(b)
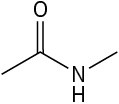
(c)
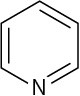
(d)
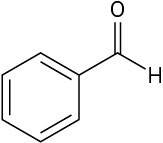
Problem 1.59
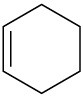
Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with HCl and draw the resulting carbocation.
(a)
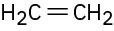
(b)
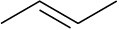
(c)
Electronegativity and Dipole Moments
Problem 1.60
Identify the most electronegative element in each of the following molecules:
(a) CH2FCl
(b) FCH2CH2CH2Br
(c) HOCH2CH2NH2
(d) CH3OCH2Li
Problem 1.61
Use the electronegativity table given in Figure 2.3 to predict which bond in each of the following pairs is more polar, and indicate the direction of bond polarity for each compound.
(a) H3C–Cl or Cl–Cl
(b) H3C–H or H–Cl
(c) HO–CH3 or (CH3)3Si–CH3
(d) H3C–Li or Li–OH
Formal Charges
Problem 1.62
Calculate the formal charges on the atoms shown in red.
(a)
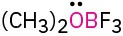
(b)
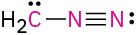
(c)
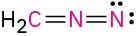
(d)
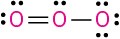
(e)
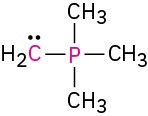
(f)
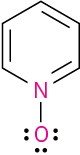
Problem 1.63
Assign formal charges to the atoms in each of the following molecules:
(a)
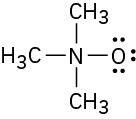
(b)
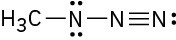
(c)
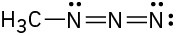
Resonance
Problem 1.64
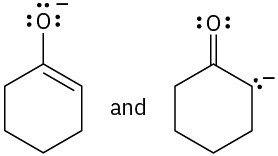
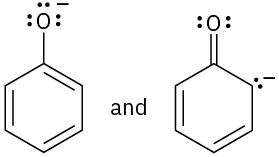
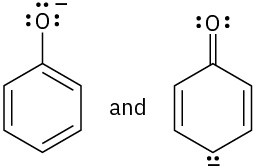
Which of the following pairs of structures represent resonance forms?
(a)
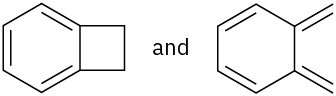
(b)
(c)
(d)
Acids and Bases
Problem 1.65
Alcohols can act either as weak acids or as weak bases, just as water can. Show the reaction of methanol, CH3OH, with a strong acid such as HCl and with a strong base such as Na+ –NH2
Problem 1.66
Draw electron-dot structures for the following molecules, indicating any unshared electron pairs. Which of the compounds are likely to act as Lewis acids and which as Lewis bases?
(a) AlBr3
(b) CH3CH2NH2
(c) BH3
(d) HF
(e) CH3SCH3
(f) TiCl4
Problem 1.67
Write the products of the following acid–base reactions:
(a) CH3OH + H2SO4 ⇄ ?
(b) CH3OH + NaNH2 ⇄ ?
(c) CH3NH3+ Cl– + NaOH ⇄ ?
Problem 1.68
Rank the following substances in order of increasing acidity:
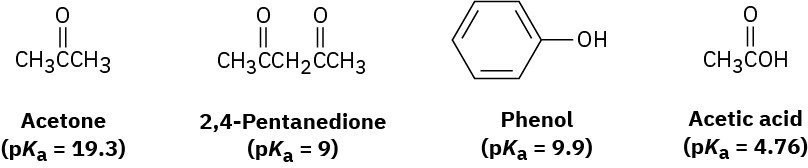
Problem 1.69
Which, if any, of the substances in Problem 1.68 is a strong enough acid to react almost completely with NaOH? (The pKa of H2O is 15.74.)
Problem 1.70
The ammonium ion (NH4+, pKa = 9.25) has a lower pKa than the methylammonium ion (CH3NH3+, pKa = 10.66). Which is the stronger base, ammonia (NH3) or methylamine (CH3NH2)? Explain.
Problem 1.71
Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electron flow.
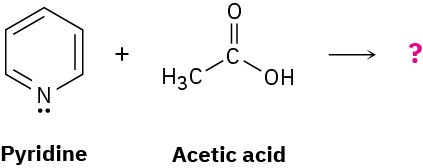
Problem 1.72
Sodium bicarbonate, NaHCO3, is the sodium salt of carbonic acid (H2CO3), pKa = 6.37. Which of the substances shown in Problem 1.68 will react significantly with sodium bicarbonate?
General Problems
Problem 1.73
Why do you suppose no one has ever been able to make cyclopentyne as a stable molecule?

Problem 1.74
Allene, H2C=C=CH2, has two adjacent double bonds. Draw a picture showing the orbitals involved in the σ and π bonds of allene. Is the central carbon atom sp2– or sp-hybridized? What about the hybridization of the terminal carbons? What shape do you predict for allene?
Problem 1.75
Allene (see Problem 1-74) is structurally related to carbon dioxide, CO2. Draw a picture showing the orbitals involved in the σ and π bonds of CO2, and identify the likely hybridization of carbon.
Problem 1.76
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicated atoms.
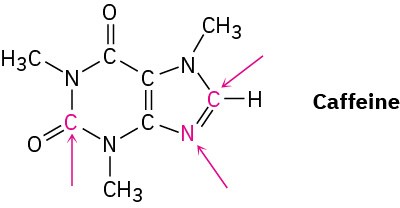
Problem 1.77
Most stable organic species have tetravalent carbon atoms, but species with trivalent carbon atoms also exist. Carbocations are one such class of compounds.

(a) How many valence electrons does the positively charged carbon atom have?
(b) What hybridization do you expect this carbon atom to have?
(c) What geometry is the carbocation likely to have?
Problem 1.78
A carbanion is a species that contains a negatively charged, trivalent carbon.

(a) What is the electronic relationship between a carbanion and a trivalent nitrogen compound such as NH3?
(b) How many valence electrons does the negatively charged carbon atom have?
(c) What hybridization do you expect this carbon atom to have?
(d) What geometry is the carbanion likely to have?
Problem 1.79
Two different substances have the formula C4H10. Draw both, and tell how they differ.
Problem 1.80
Two different substances have the formula C3H6. Draw both, and tell how they differ.
Problem 1.81
Two different substances have the formula C2H6O. Draw both, and tell how they differ.
Problem 1.82
Three different substances contain a carbon–carbon double bond and have the formula C4H8. Draw them, and tell how they differ.
Problem 1.83
Among the most common over-the-counter drugs you might find in a medicine cabinet are mild pain relievers such ibuprofen (Advil, Motrin), naproxen (Aleve), and acetaminophen (Tylenol).
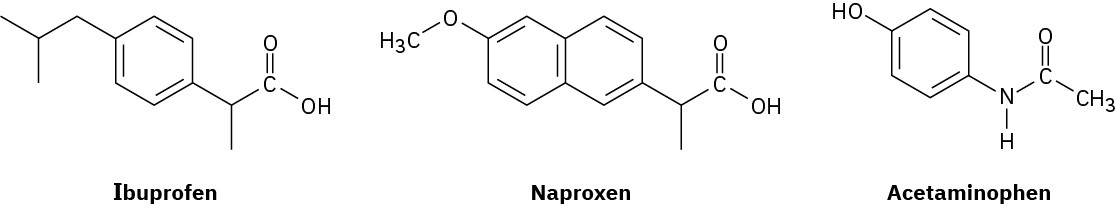
(a) How many sp3-hybridized carbons does each molecule have?
(b) How many sp2-hybridized carbons does each molecule have?
(c) What similarities can you see in their structures?
Problem 1.84
Identify the acids and bases in the following reactions:
(a)
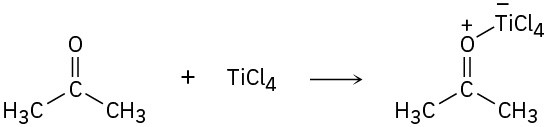
(b)
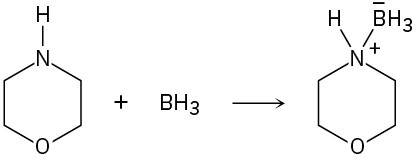
Problem 1.85
Which of the following pairs represent resonance structures?
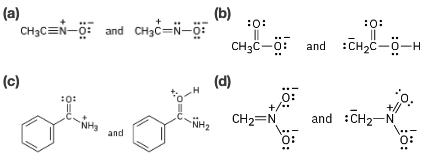
Problem 1.86
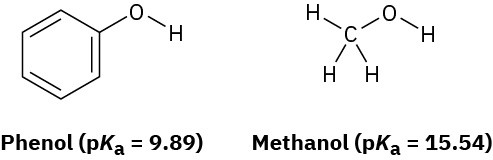
Phenol, C6H5OH, is a stronger acid than methanol, CH3OH, even though both contain an O–H bond. Draw the structures of the anions resulting from loss of H+ from phenol and methanol, and use resonance structures to explain the difference in acidity.
Problem 1.87
Thiamin diphosphate (TPP), a derivative of vitamin B1 required for glucose metabolism, is a weak acid that can be deprotonated by a base. Assign formal charges to the appropriate atoms in both TPP and its deprotonation product.
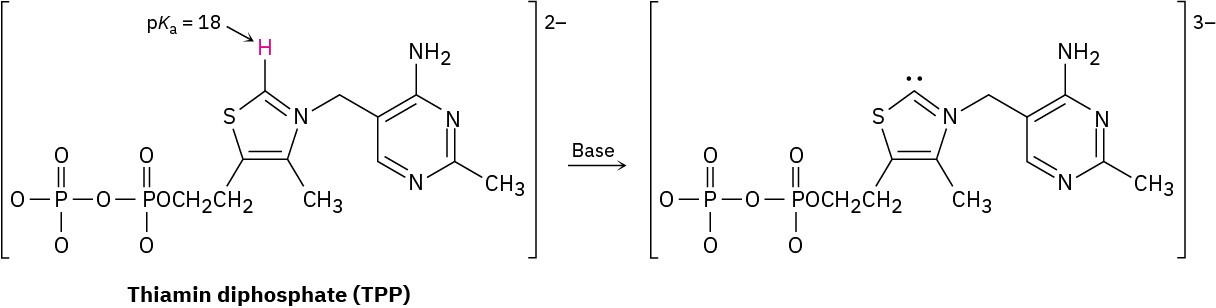
Problem 1.88
Use the pKa table in Appendix B to determine in which direction the equilibrium is favored.
(a)
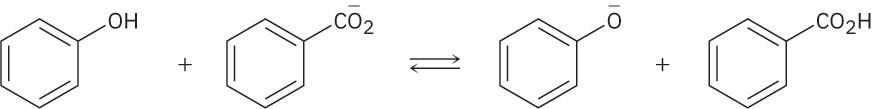
(b)

(c)


