Additional Problems 10
Visualizing Chemistry
Problem 10.11
Each of the following substances can be prepared by a nucleophilic addition reaction between an aldehyde or ketone and a nucleophile. Identify the reactants from which each was prepared. If the substance is an acetal, identify the carbonyl compound and the alcohol; if it is an imine, identify the carbonyl compound and the amine; and so forth.
(a)
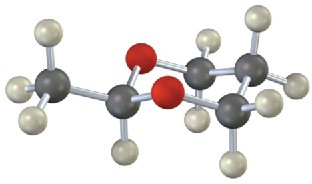
(b)
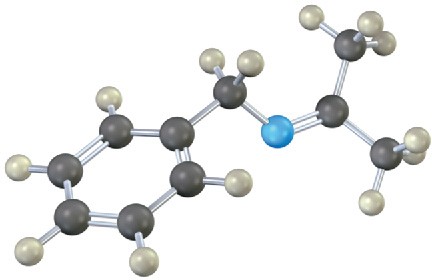
(c)
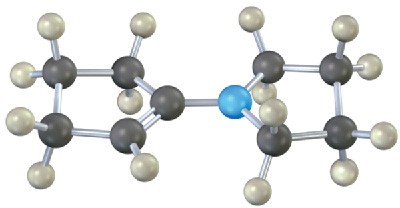
(d)
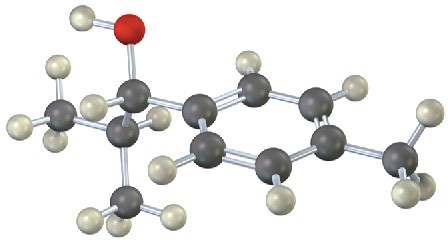
Problem 10.12
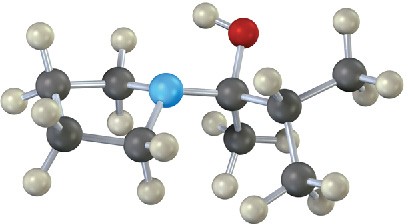
The following molecular model represents a tetrahedral intermediate resulting from addition of a nucleophile to an aldehyde or ketone. Identify the reactants, and write the structure of the final product when the nucleophilic addition reaction is complete.
Mechanism Problems
Problem 10.13
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
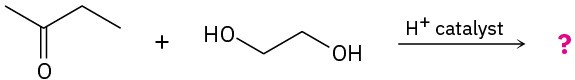
(b)
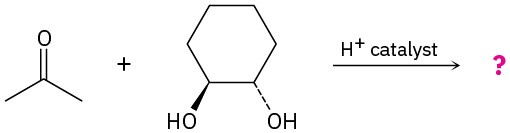
Problem 10.14
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
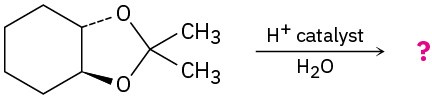
(b)
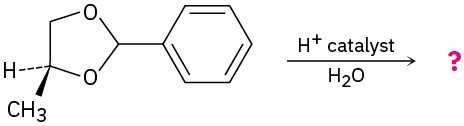
Problem 10.15
Predict the product(s) and propose a mechanism for the following reaction:
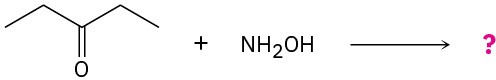
Problem 10.16
Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:
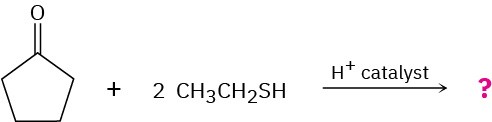
Naming Aldehydes and Ketones
Problem 10.17
Draw structures corresponding to the following names:
(a) Bromoacetone
(b) (S)-2-Hydroxypropanal
(c) 2-Methyl-3-heptanone
(d) (2S,3R)-2,3,4-Trihydroxybutanal
(e) 2,2,4,4-Tetramethyl-3-pentanone
(f) 4-Methyl-3-penten-2-one
(g) Butanedial
(h) 3-Phenyl-2-propenal
(i) 6,6-Dimethyl-2,4-cyclohexadienone
(j) p-Nitroacetophenone
Problem 10.18
Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?
Problem 10.19
Give IUPAC names for the following compounds:
(a)
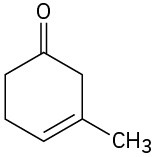
(b)
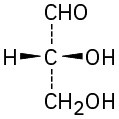
(c)
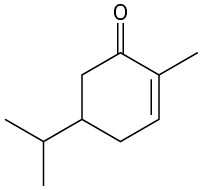
(d)
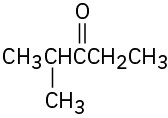
(e)
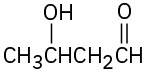
(f)
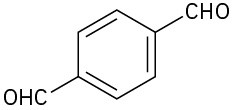
Problem 10.20
Draw structures of compounds that fit the following descriptions:
(a) An aromatic ketone, C9H10O
(b) A diene aldehyde, C7H8O
Reactions of Aldehydes and Ketones
Problem 10.21
Predict the products of the reaction of (1) phenylacetaldehyde and (2) acetophenone with the following reagents:
(a) NaBH4, then H3O+
(b) Na2Cr2O7, H2SO4
(c) NH2OH, HCl catalyst
(d) CH3MgBr, then H3O+
(e) 2 CH3OH, HCl catalyst
(f) HCN, KCN
Problem 10.22
How would you use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds?
(a) 2-Pentanol
(b) 1-Butanol
(c) 1-Phenylcyclohexanol
(d) Diphenylmethanol
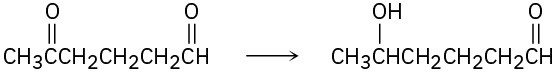
Problem 10.23
How might you carry out the following selective transformations? One of the two schemes requires a protection step. (Recall from Section 10.4 that aldehydes are more reactive than ketones toward nucleophilic addition.)
(a)
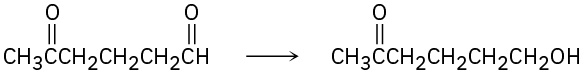
(b)
Problem 10.24
Carvone is the major constituent of spearmint oil. What products would you expect from reaction of carvone with the following reagents?
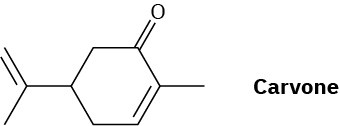
(a) LiAlH4, then H3O+
(b) C6H5MgBr, then H3O+
(c) H2/Pd
(d) HOCH2CH2OH, HCl
General Problems
Problem 10.25
When 4-hydroxybutanal is treated with methanol in the presence of an acid catalyst, 2-methoxytetrahydrofuran is formed. Explain.
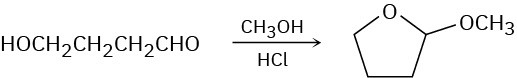
Problem 10.26
Reaction of 2-butanone with HCN yields a chiral product. What stereochemistry does the product have? Is it optically active?

