Additional Problems 11: Carboxylic Acid Derivatives
Visualizing Chemistry
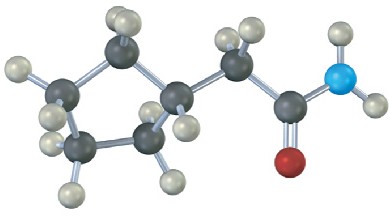
Problem 11.60
Name the following compounds:
(a)
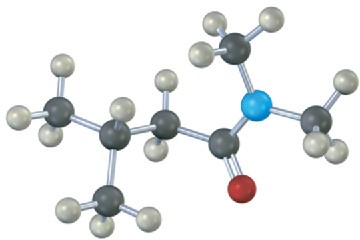
(b)
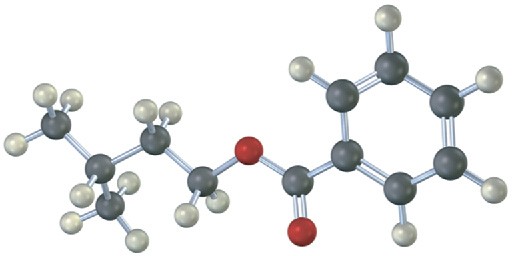
Problem 11.61
How would you prepare the following compounds starting with an appropriate carboxylic acid and any other reagents needed? (reddish brown = Br.)
(a)
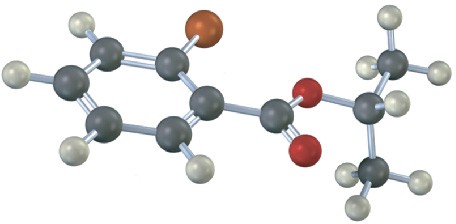
(b)
Problem 11.62
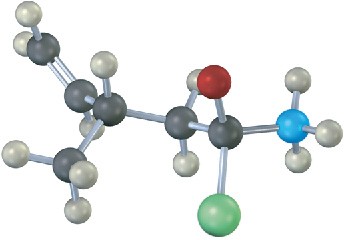
The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting acid derivative, and the ultimate product (green = Cl).
Problem 11.63
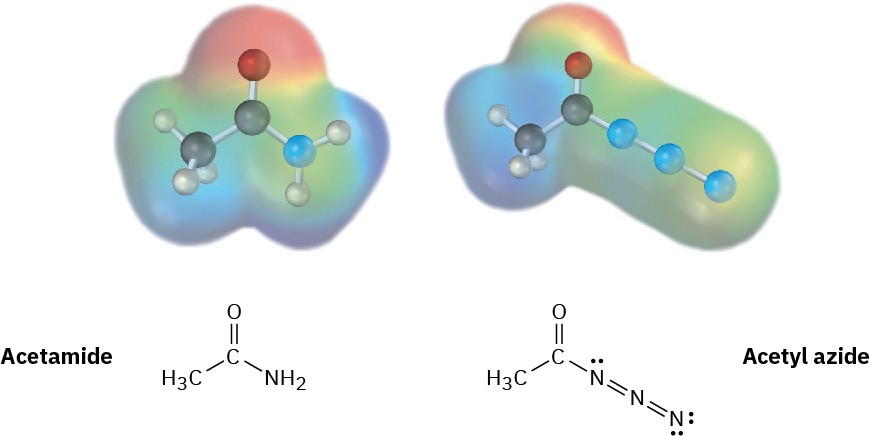
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions? Explain.
Mechanism Problems
Problem 11.64
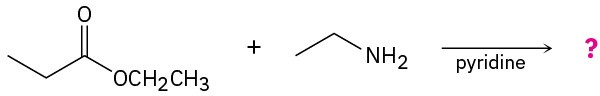
Predict the product(s) and write the mechanism for the following reactions:
(a)
(b)
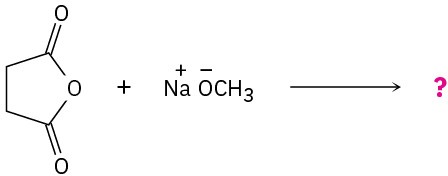
Problem 11.65
Predict the product(s) for the following reactions:
(a)
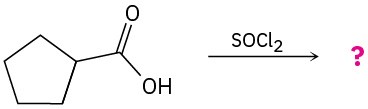
(b)
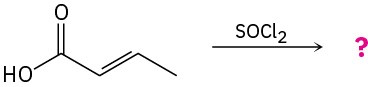
Problem 11.66
Predict the product(s) for each of the following reactions:
(a)
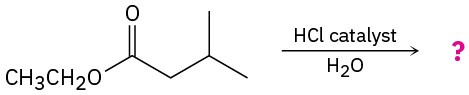
(b)
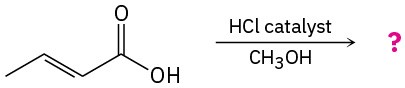
Problem 11.67
Predict the product(s) for the following reactions:
(a)
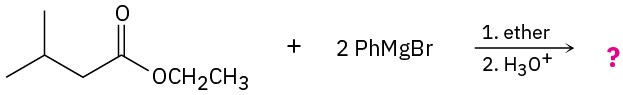
(b)
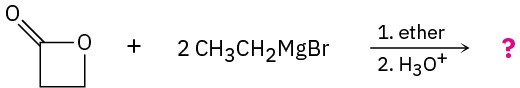
Problem 11.68
Fats are biosynthesized from glycerol 3-phosphate and fatty-acyl CoA’s by a reaction sequence that begins with the following step. Show the mechanism of the reaction.
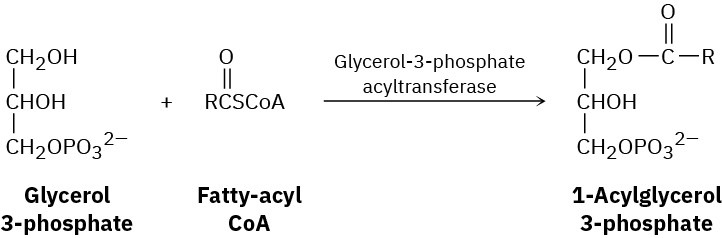
Problem 11.69
Bacteria typically develop a resistance to penicillins and other β-lactam antibiotics (see Chemistry Matters at the end of this chapter) due to bacterial synthesis of β-lactamase enzymes. Tazobactam, however, is able to inhibit the activity of the β-lactamase by trapping it, thereby preventing a resistance from developing.
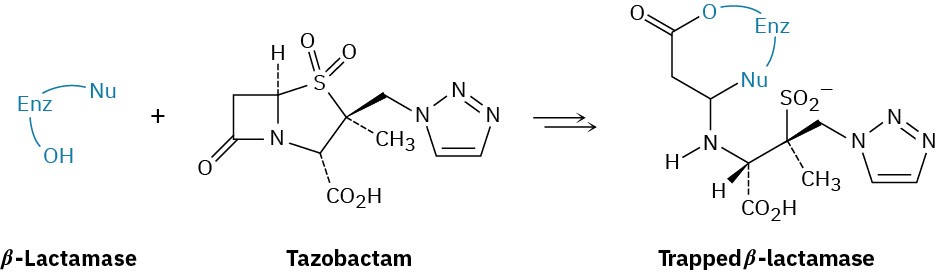
The first step in trapping is reaction of a hydroxyl group on the β-lactamase to open the β– lactam ring of tazobactam. Show the mechanism.
Problem 11.70
In the iodoform reaction, a triiodomethyl ketone reacts with aqueous NaOH to yield a carboxylate ion and iodoform (triiodomethane). Propose a mechanism for this reaction.
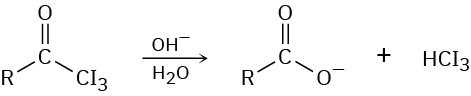
Naming Carboxylic Acid Derivatives
Problem 11.71
Give IUPAC names for the following compounds:
(a)
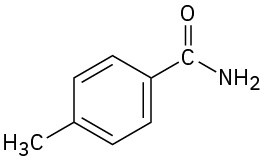
(b)
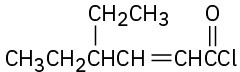
(c)
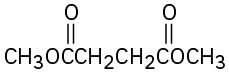
(d)
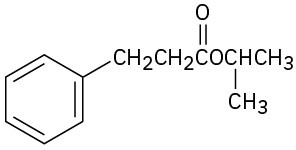
(e)
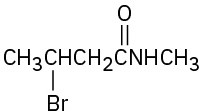
(f)
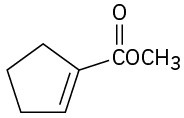
(g)
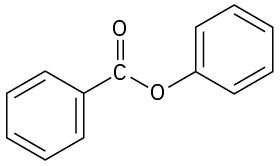
(h)
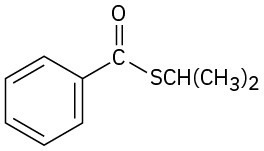
Problem 11.72
Draw structures corresponding to the following names:
(a) p-Bromophenylacetamide
(b) m-Benzoylbenzamide
(c) 2,2-Dimethylhexanamide
(d) Cyclohexyl cyclohexanecarboxylate
(e) Ethyl 2-cyclobutenecarboxylate
(f) Succinic anhydride
Problem 11.73
Draw and name compounds that meet the following descriptions:
(a) Three acid chlorides having the formula C6H9ClO
(b) Three amides having the formula C7H11NO
Nucleophilic Acyl Substitution Reactions
Problem 11.74
Predict the product, if any, of reaction between propanoyl chloride and the following reagents:
(a) LiAlH4, then H3O+
(b) CH3MgBr, then H3O+
(c) H3O+
(d) Cyclohexanol
(e) Aniline
(f) CH3CO2– Na+
Problem 11.75
Answer Problem 11.74 for reaction of the listed reagents with methyl propanoate.
Problem 11.76
Answer Problem 11.74 for reaction of the listed reagents with propanamide.
Problem 11.77
How might you prepare the following compounds from butanoic acid?
(a) 1-Butanol
(b) Butanal
(c) 1-Bromobutane
(d) Pentanenitrile
(e) Butene
(f) N-Methylpentanamide
(g) 2-Hexanone
(h) Butylbenzene
(i) Butanenitrile
Problem 11.78
Predict the product(s) of the following reactions:
(a)
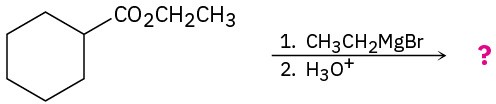
(b)
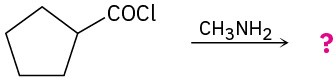
(c)
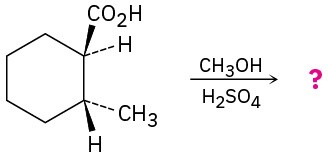
(d)
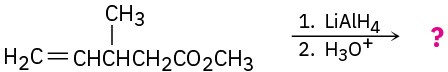
(e)
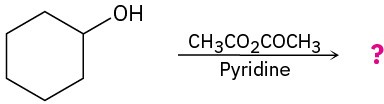
(f)
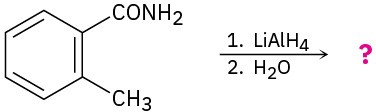
(g)
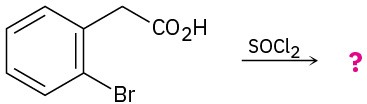
Problem 11.79
The following reactivity order has been found for the saponification of alkyl acetates by aqueous NaOH. Explain.
CH3CO2CH3 > CH3CO2CH2CH3 > CH3CO2CH(CH3)2 > CH3CO2C(CH3)3
Problem 11.80
Explain the observation that attempted Fischer esterification of 2,4,6-trimethylbenzoic acid with methanol and HCl is unsuccessful. No ester is obtained, and the acid is recovered unchanged. What alternative method of esterification might be successful?
Problem 11.81
Outline methods for the preparation of acetophenone (phenyl methyl ketone) starting from the following:
(a) Benzene
(b) Benzonitrile
(c) Styrene
Problem 11.82
Treatment of 5-aminopentanoic acid with DCC (dicyclohexylcarbodiimide) yields a lactam. Show the structure of the product of the reaction.
Problem 11.83
tert-Butoxycarbonyl azide, a reagent used in protein synthesis, is prepared by treating tert-butoxycarbonyl chloride with sodium azide. Propose a mechanism for this reaction.
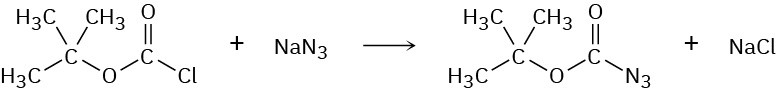
Step-Growth Polymers
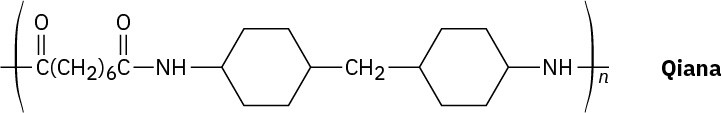
Problem 11.84
Qiana, a polyamide fiber with a silky texture, has the following structure. What are the monomer units used in the synthesis of Qiana?
Problem 11.85
What is the structure of the polymer produced by treatment of β-propiolactone with a small amount of hydroxide ion?
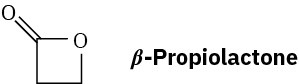
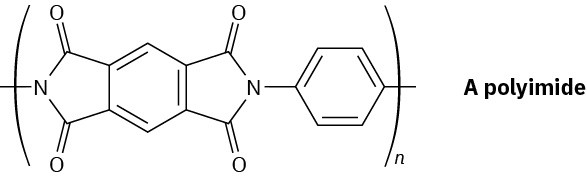
Problem 11.86
Polyimides with the structure shown are used as coatings on glass and plastics to improve scratch resistance. How would you synthesize a polyimide?
General Problems
Problem 11.87
The following reactivity order has been found for the basic hydrolysis of p-substituted methyl benzoates:
Y=NO2 > Br > H > CH3 > OCH3
How can you explain this reactivity order? Where would you expect Y=C≡N, Y=CHO, and Y=NH2 to be in the reactivity list?
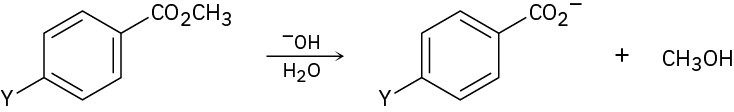
Problem 11.88
One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2.
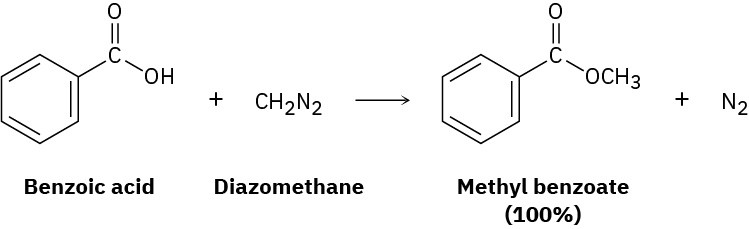
The reaction occurs in two steps: (1) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+.
(a) Draw two resonance structures of diazomethane, and account for step 1.
(b) What kind of reaction occurs in step 2?
Problem 11.89
When an amide is formed from an acid chloride or an anhydride, two equivalents of base are required. However, when an ester is used as the starting material, only one equivalent of base is needed. Explain this reactivity in terms of basicity of the leaving groups.

