Additional Problems 2
Visualizing Chemistry
Problem 2.32
Identify the functional groups in the following substances, and convert each drawing into a molecular formula (red = O, blue = N).
(a)
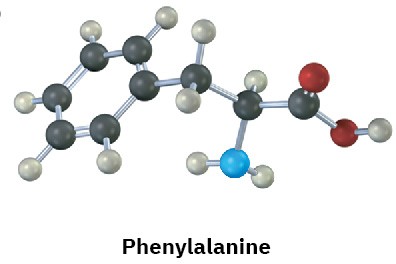
(b)
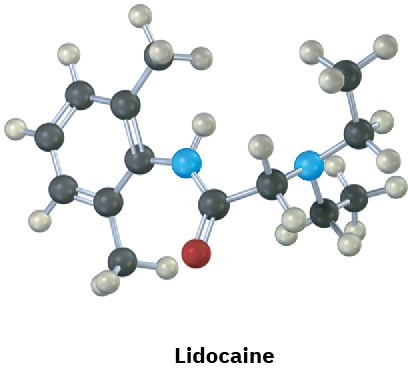
Problem 2.33
Give IUPAC names for the following alkanes, and convert each drawing into a skeletal structure.
(a)
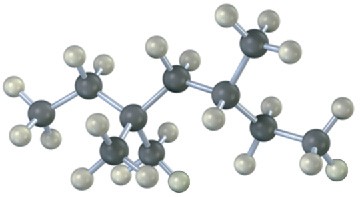
(b)
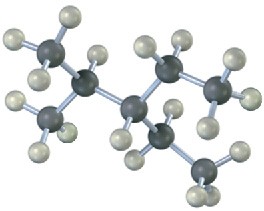
(c)
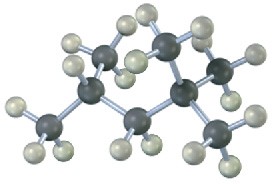
(d)
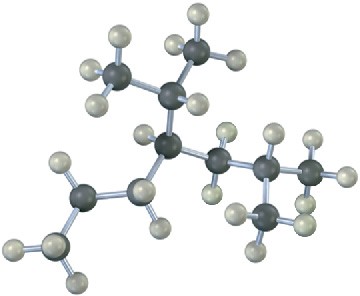
Problem 2.34
Draw a Newman projection along the C2–C3 bond of the following conformation of 2-butanol.
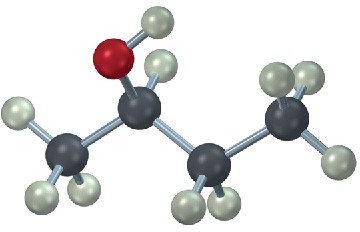
Problem 2.35
Name the following cycloalkanes:
(a)
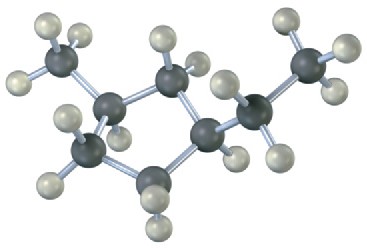
(b)
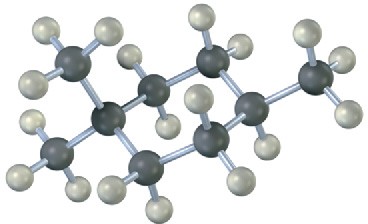
Problem 2.36
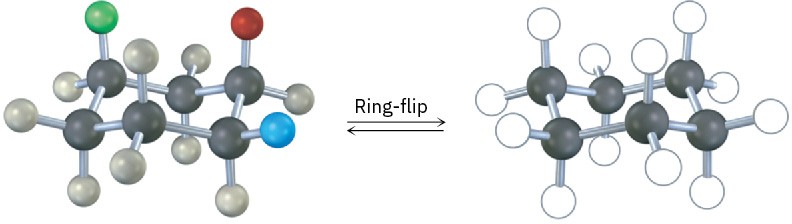
A trisubstituted cyclohexane with three substituents—red, green, and blue—undergoes a ring-flip to its alternate chair conformation. Identify each substituent as axial or equatorial, and show the positions occupied by the three substituents in the ring-flipped form.
Problem 2.37
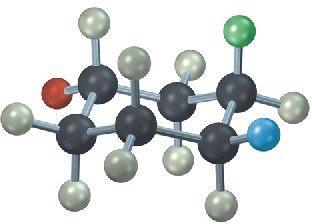
The following cyclohexane derivative has three substituents—red, green, and blue. Identify each substituent as axial or equatorial, and identify each pair of relationships (red–blue, red–green, and blue–green) as cis or trans.
Problem 2.38
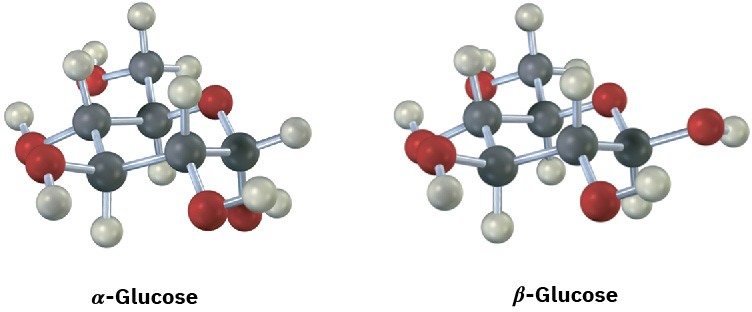
Glucose exists in two forms having a 36:64 ratio at equilibrium. Draw a skeletal structure of each, describe the difference between them, and tell which of the two you think is more stable (red = O).
Functional Groups
Problem 2.39
Locate and identify the functional groups in the following molecules.
(a)
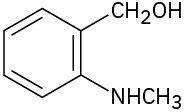
(b)
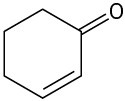
(c)
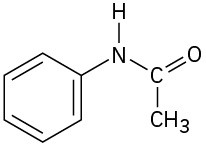
(d)
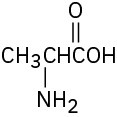
(e)
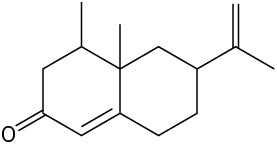
(f)
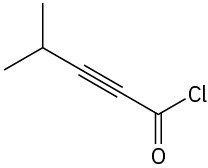
Problem 2.40
Propose structures that meet the following descriptions:
(a) A ketone with five carbons
(b) A four-carbon amide
(c) A five-carbon ester
(d) An aromatic aldehyde
(e) A keto ester
(f) An amino alcohol
Problem 2.41
Propose structures for the following:
(a) A ketone, C4H8O
(b) A nitrile, C5H9N
(c) A dialdehyde, C4H6O2
(d) A bromoalkene, C6H11Br
(e) An alkane, C6H14
(f) cyclic saturated hydrocarbon, C6H12
(g) A diene (dialkene), C5H8
(h) A keto alkene, C5H8O
Problem 2.42
Predict the hybridization of the carbon atom in each of the following functional groups:
(a) Ketone
(b) Nitrile
(c) Carboxylic acid
Problem 2.43
Draw the structures of the following molecules:
(a) Biacetyl, C4H6O2, a substance with the aroma of butter; it contains no rings or carbon– carbon multiple bonds.
(b) Ethylenimine, C2H5N, a substance used in the synthesis of melamine polymers; it contains no multiple bonds.
(c) Glycerol, C3H8O3, a substance isolated from fat and used in cosmetics; it has an –OH group on each carbon.
Isomers
Problem 2.44
Draw structures that meet the following descriptions (there are many possibilities):
(a) Three isomers with the formula C8H18
(b) Two isomers with the formula C4H8O2
Problem 2.45
Draw structures of the nine isomers of C7H16.
Problem 2.46
In each of the following sets, which structures represent the same compound and which represent different compounds?
(a)
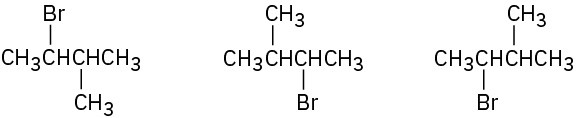
(b)
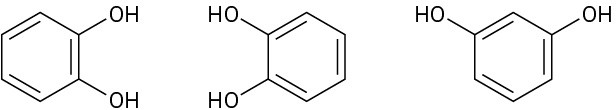
(c)
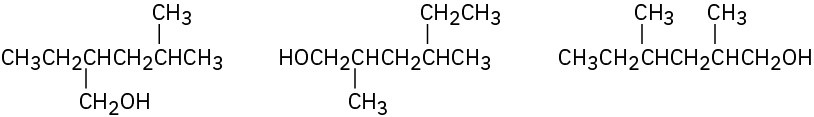
Problem 2.47
Seven constitutional isomers have the formula C4H10O. Draw as many as you can.
Problem 2.48
Draw as many compounds as you can that fit the following descriptions:
(a) Alcohols with formula C4H10O
(b) Amines with formula C5H13N
(c) Ketones with formula C5H10O
(d) Aldehydes with formula C5H10O
(e) Esters with formula C4H8O2
(f) Ethers with formula C4H10O
Problem 2.49
Draw compounds that contain the following:
(a) A primary alcohol
(b) A tertiary nitrile
(c) A secondary thiol
(d) Both primary and secondary alcohols
(e) An isopropyl group
(f) A quaternary carbon
Cycloalkane Isomers
Problem 2.50
Draw the five cycloalkanes with the formula C5H10.
Problem 2.51
Give IUPAC names for the following compounds.
(a)
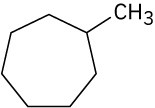
(b)
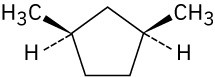
(c)
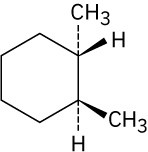
(d)
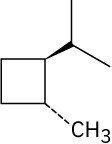
(e)
Problem 2.52
Draw a stereoisomer of trans-1,3-dimethylcyclobutane.
Problem 2.53
Tell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated.
(a) cis-1,3-Dibromocyclohexane and trans-1,4-dibromocyclohexane
(b) 2,3-Dimethylhexane and 2,3,3-trimethylpentane
(c)

Problem 2.54
Draw three isomers of trans-1,2-dichlorocyclobutane, and label them as either constitutional isomers or stereoisomers.
Problem 2.55
Identify each pair of relationships among the –OH groups in glucose (red–blue, red–green, red–black, blue–green, blue–black, green–black) as cis or trans.
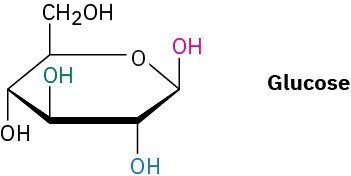
Naming Compounds
Problem 2.56
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 2.57
Draw and name all monochloro derivatives of 2,5-dimethylhexane, C8H17Cl.
Problem 2.58
Draw structures for the following:
(a) 2-Methylheptane
(b) 4-Ethyl-2,2-dimethylhexane
(c) 4-Ethyl-3,4-dimethyloctane
(d) 2,4,4-Trimethylheptane
(e) 3,3-Diethyl-2,5-dimethylnonane
(f) 4-Isopropyl-3-methylheptane
Problem 2.59
Draw a compound that:
(a) Has only primary and tertiary carbons
(b) Has no secondary or tertiary carbons
(c) Has no secondary or tertiary carbons
Problem 2.60
Draw a compound that:
(a) Has nine primary hydrogens
(b) Has only primary hydrogens
Problem 2.61
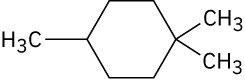
Give IUPAC names for the following compounds:
(a)
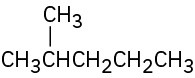
(b)
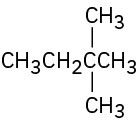
(c)
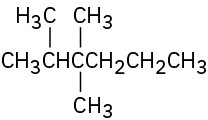
(d)
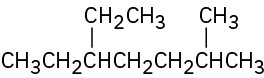
(e)
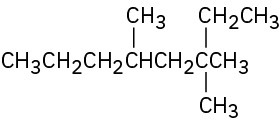
(f)
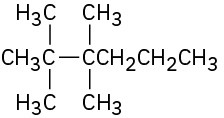
Problem 2.62
Name the five isomers of C6H14.
Problem 2.63
Explain why each of the following names is incorrect:
(a) 2,2-Dimethyl-6-ethylheptane
(b) 4-Ethyl-5,5-dimethylpentane
(c) 3-Ethyl-4,4-dimethylhexane
(d) 5,5,6-Trimethyloctane
(e) 2-Isopropyl-4-methylheptane
Problem 2.64
Propose structures and give IUPAC names for the following:
(a) A diethyldimethylhexane
(b) A (3-methylbutyl)-substituted alkane
Conformations
Problem 2.65
Consider 2-methylbutane (isopentane). Sighting along the C2–C3 bond:
(a) Draw a Newman projection of the most stable conformation.
(b) Draw a Newman projection of the least stable conformation.
Problem 2.66
Draw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.
Problem 2.67
Draw the most stable conformation of 1,4-dichlorobutane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.
General Problems
Problem 2.68
For each of the following compounds, draw an isomer that has the same functional groups.
(a)
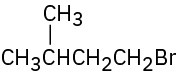
(b)
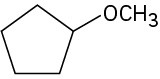
(c)
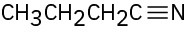
(d)
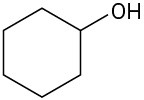
(e)
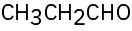
(f)
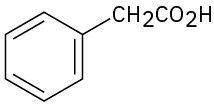
Problem 2.69
Malic acid, C4H6O5, has been isolated from apples. Because this compound reacts with 2 molar equivalents of base, it is a dicarboxylic acid.
(a) Draw at least five possible structures.
(b) If malic acid is a secondary alcohol, what is its structure?
Problem 2.70
Increased substitution around a bond leads to increased strain. Take the four substituted butanes listed below, for example. For each compound, sight along the C2–C3 bond and draw Newman projections of the most stable and least stable conformations.
(a) 2-Methylbutane
(b) 2,2-Dimethylbutane
(c) 2,3-Dimethylbutane
(d) 2,2,3-Trimethylbutane
Problem 2.71
The cholesterol-lowering agents called statins, such as simvastatin (Zocor) and pravastatin (Pravachol), are among the most widely prescribed drugs in the world, with annual sales estimated at approximately $25 billion. Identify the functional groups in both, and tell how the two substances differ.
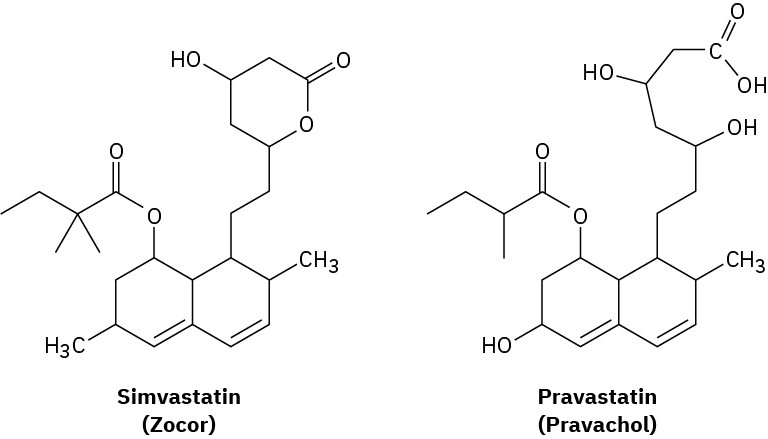
Problem 2.72
There are two isomeric substances, both named 1,2-dimethylcyclohexane. Explain.

Problem 2.73
The statin drugs, such as simvastatin (Zocor), pravastatin (Pravachol), and atorvastatin (Lipitor) are the most widely prescribed drugs in the world.
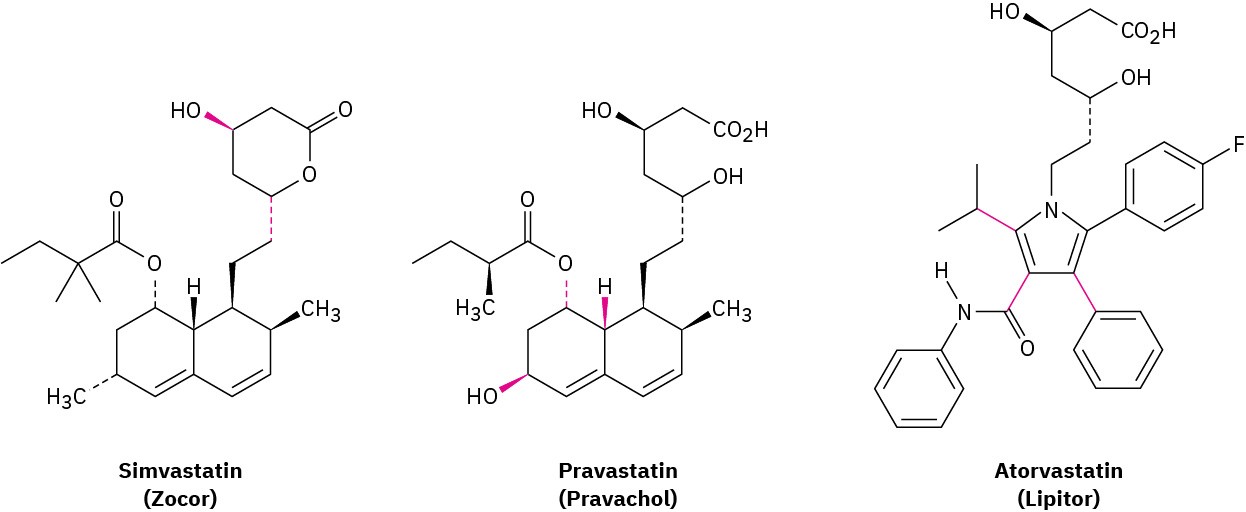
(a) Are the two indicated bonds on simvastatin cis or trans?
(b) What are the cis/trans relationships among the three indicated bonds on pravastatin?
(c) Why can’t the three indicated bonds on atorvastatin be identified as cis or trans?
Problem 2.74
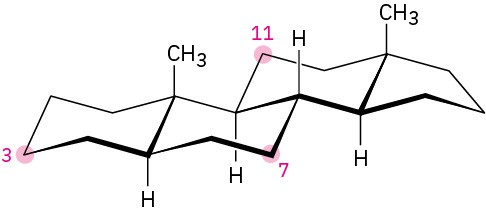
Tell whether each of the following substituents on a steroid is axial or equatorial. A substituent that is “up” is on the top side of the molecule as drawn, and a substituent that is “down” is on the bottom side.
(a) Substituent up at C3
(b) Substituent down at C7
(c) Substituent down at C11
Cycloalkane Conformation and Stability
Problem 2.75
A 1,2-cis disubstituted cyclohexane, such as cis-1,2-dichlorocyclohexane, must have one group axial and one group equatorial. Explain.
Problem 2.76
A 1,2-trans disubstituted cyclohexane must have either both groups axial or both groups equatorial. Explain.
Problem 2.77
Assume that you have a variety of cyclohexanes substituted in the positions indicated. Identify the substituents as either axial or equatorial. For example, a 1,2-cis relationship means that one substituent must be axial and one equatorial, whereas a 1,2-trans relationship means that both substituents are axial or both are equatorial.
(a) 1,3-Trans disubstituted
(b) 1,4-Cis disubstituted
(c) 1,3-Cis disubstituted
(d) 1,5-Trans disubstituted
(e) 1,5-Cis disubstituted
(f) 1,6-Trans disubstituted
Cyclohexane Conformational Analysis
Problem 2.78
Draw the two chair conformations of trans-1-chloro-2-methylcyclohexane. Which is more stable?

