Additional Problems 3
Visualizing Chemistry
Problem 3.18
Which of the following structures are identical? (Green = Cl.)
(a)
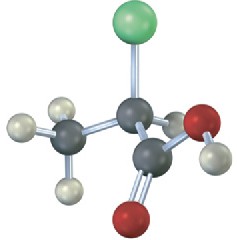
(b)
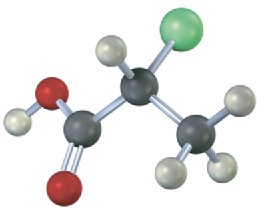
(c)
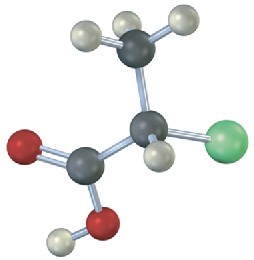
(d)
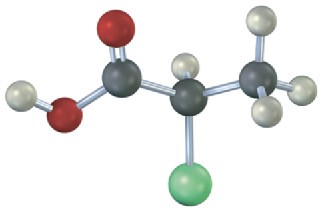
Problem 3.19
Assign R or S configurations to the chirality centers in the following molecules (blue = N):
(a)
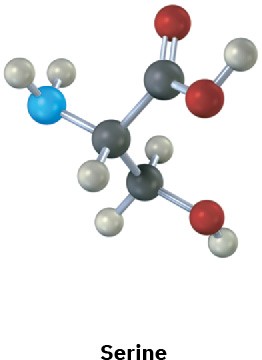
(b)
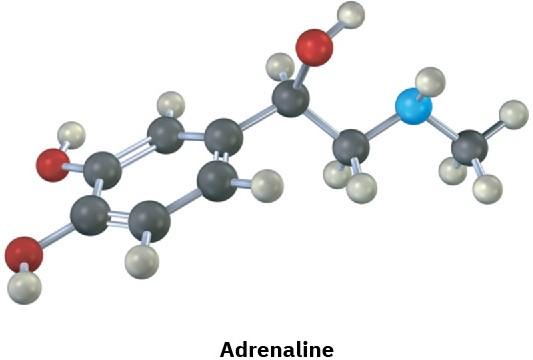
Problem 3.20
Which, if any, of the following structures represent meso compounds? (Blue = N, green = Cl.)
(a)
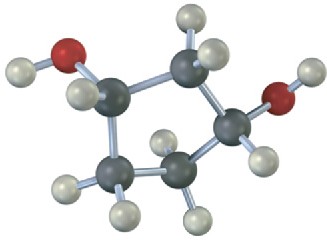
(b)
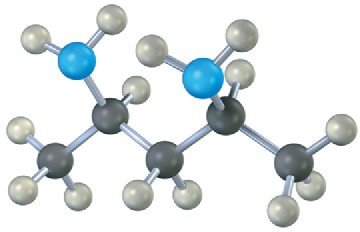
(c)
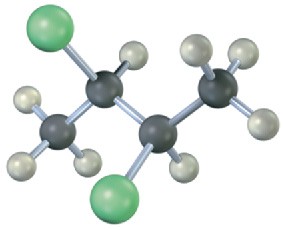
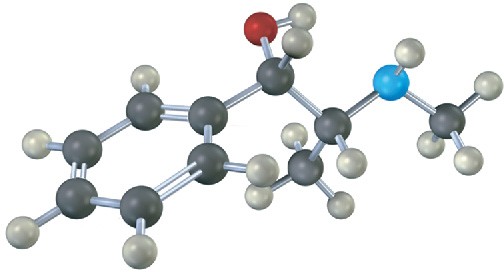
Problem 3.21
Assign R or S configuration to each chirality center in pseudoephedrine, an over-the- counter decongestant found in cold remedies (blue = N).
Problem 3.22
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
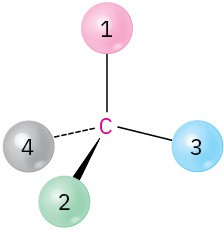
(b)
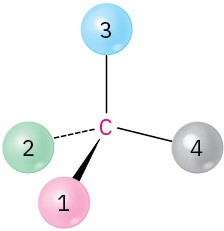
(c)
Chirality and Optical Activity
Problem 3.23
Which of the following objects are chiral?
(a) A basketball
(b) A fork
(c) A wine glass
(d) A golf club
(e) A spiral staircase
(f) A snowflake
Problem 3.24
Which of the following compounds are chiral? Draw them, and label the chirality centers.
(a) 2,4-Dimethylheptane
(b) 5-Ethyl-3,3-dimethylheptane
(c) cis-1,4-Dichlorocyclohexane
Problem 3.25
Draw chiral molecules that meet the following descriptions:
(a) A chloroalkane, C5H11Cl
(b) An alcohol, C6H14O
(c) An alkene, C6H12
(d) An alkane, C8H18
Problem 3.26
Eight alcohols have the formula C5H12O. Draw them. Which are chiral?
Problem 3.27
Draw compounds that fit the following descriptions:
(a) A chiral alcohol with four carbons
(b) A chiral carboxylic acid with the formula C5H10O2
(c) A compound with two chirality centers
(d) A chiral aldehyde with the formula C3H5BrO
Problem 3.28
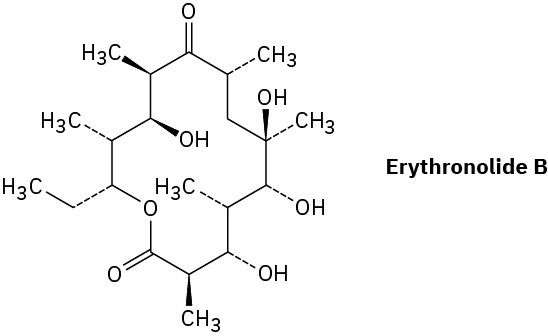
Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How many chirality centers does erythronolide B have? Identify them.
Assigning Configuration to Chirality Centers
Problem 3.29
Which of the following pairs of structures represent the same enantiomer, and which represent different enantiomers?
(a)
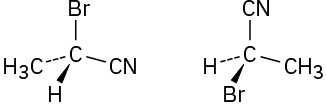
(b)
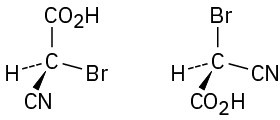
(c)
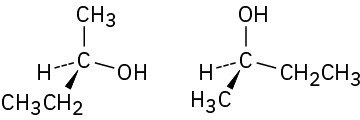
(d)
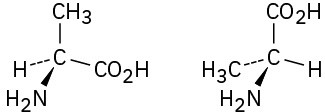
Problem 3.30
What is the relationship between the specific rotations of (2R,3R)-dichloropentane and (2S,3S)-dichloropentane? Between (2R,3S)-dichloropentane and (2R,3R)-dichloropentane?
Problem 3.31
What is the stereochemical configuration of the enantiomer of (2S,4R)-2,4-octanediol? (A diol is a compound with two –OH groups.)
Problem 3.32
What are the stereochemical configurations of the two diastereomers of (2S,4R)-2,4-octanediol?
Problem 3.33
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
(a)
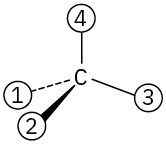
(b)
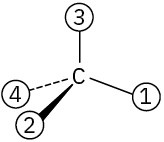
(c)
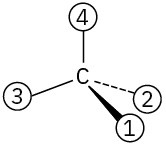
Problem 3.34
Assign Cahn–Ingold–Prelog rankings to the following sets of substituents:
(a)
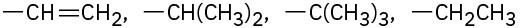
(b)
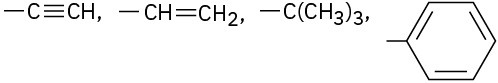
(c)
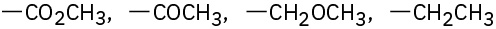
(d)
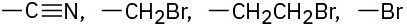
Problem 3.35
Assign R or S configurations to each chirality center in the following molecules:
(a)
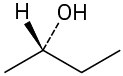
(b)
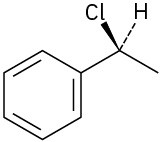
(c)
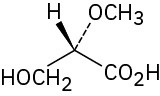
Problem 3.36
Assign R or S configuration to each chirality center in the following molecules:
(a)
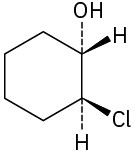
(b)
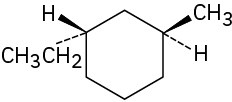
(c)
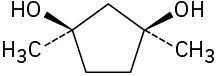
Problem 3.37
Draw tetrahedral representations of the following molecules:
(a) (S)-2-Chlorobutane
(b) (R)-3-Chloro-1-pentene [H2C=CHCH(Cl)CH2CH3]
Problem 3.38
Assign R or S configuration to each chirality center in the following molecules:
(a)
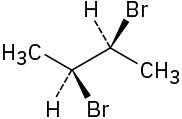
(b)
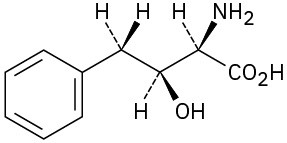
Problem 3.39
Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C).
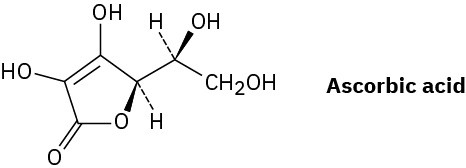
Problem 3.40
Assign R or S stereochemistry to the chirality centers in the following Newman projections:
(a)
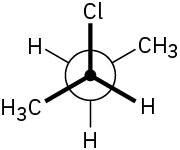
(b)
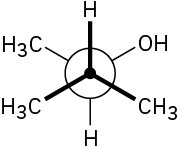
Problem 3.41
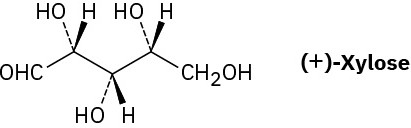
Xylose is a common sugar found in many types of wood, including maple and cherry. Because it is much less prone to cause tooth decay than sucrose, xylose has been used in candy and chewing gum. Assign R or S configurations to the chirality centers in xylose.
Meso Compounds
Problem 3.42
Draw examples of the following:
(a) A meso compound with the formula C8H18
(b) A meso compound with the formula C9H20
(c) A compound with two chirality centers, one R and the other S
Problem 3.43
Ribose, an essential part of ribonucleic acid (RNA), has the following structure:
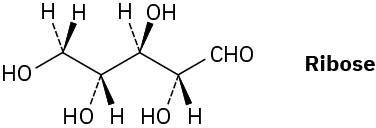
(a) How many chirality centers does ribose have? Identify them.
(b) How many stereoisomers of ribose are there?
(c) Draw the structure of the enantiomer of ribose.
(d) Draw the structure of a diastereomer of ribose.
Problem 3.44
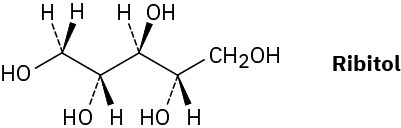
On reaction with hydrogen gas in the presence of a platinum catalyst, ribose (Problem 3.43) is converted into ribitol. Is ribitol optically active or inactive? Explain.
General Problems
Problem 3.45
Draw all possible stereoisomers of 1,2-cyclobutanedicarboxylic acid, and indicate the interrelationships. Which, if any, are optically active? Do the same for 1,3-cyclobutanedicarboxylic acid.
Problem 3.46
Draw tetrahedral representations of the following molecules:
(a) The 2S,3R enantiomer of 2,3-dibromopentane
(b) The meso form of 3,5-heptanediol
Problem 3.47
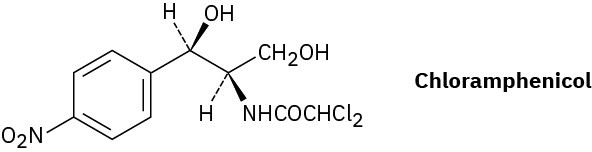
Chloramphenicol, a powerful antibiotic isolated in 1947 from the Streptomyces venezuelae bacterium, is active against a broad spectrum of bacterial infections and is particularly valuable against typhoid fever. Assign R or S configurations to the chirality centers in chloramphenicol.
Problem 3.48
How many stereoisomers of 2,4-dibromo-3-chloropentane are there? Draw them, and indicate which are optically active.
Problem 3.49
Draw both cis– and trans-1,4-dimethylcyclohexane in their more stable chair conformations.
(a) How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans– 1,4-dimethylcyclohexane?
(b) Are any of the structures chiral?
(c) What are the stereochemical relationships among the various stereoisomers of 1,4- dimethylcyclohexane?
Problem 3.50
Draw both cis– and trans-1,3-dimethylcyclohexane in their more stable chair conformations.
(a) How many stereoisomers are there of cis-1,3-dimethylcyclohexane, and how many of trans– 1,3-dimethylcyclohexane?
(b) Are any of the structures chiral?
(c) What are the stereochemical relationships among the various stereoisomers of 1,3- dimethylcyclohexane?

