Additional Problems 8
Visualizing Chemistry
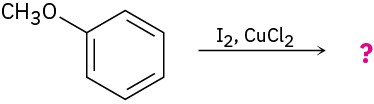
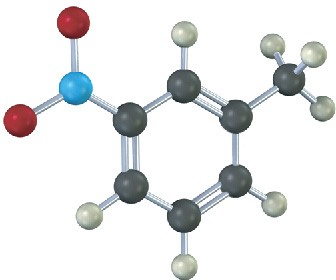
Problem 8.14
Give IUPAC names for the following substances (red = O, blue = N):
(a)
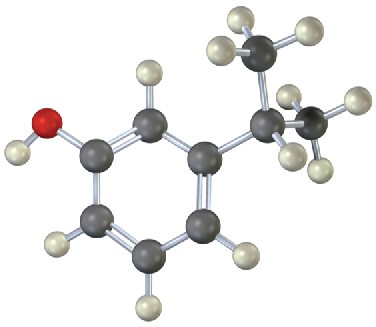
(b)
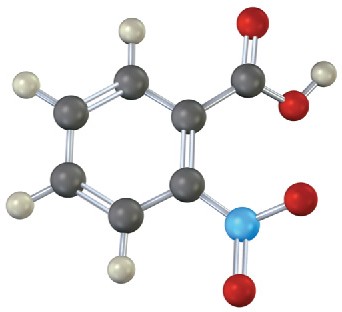
Problem 8.15
The following molecular model is that of a carbocation. Draw two resonance structures for the carbocation, indicating the positions of the double bonds.
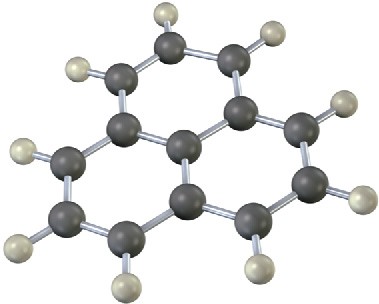
Problem 8.16
Azulene, an isomer of naphthalene, has a remarkably large dipole moment for a hydrocarbon (μ = 1.0 D). Explain, using resonance structures.
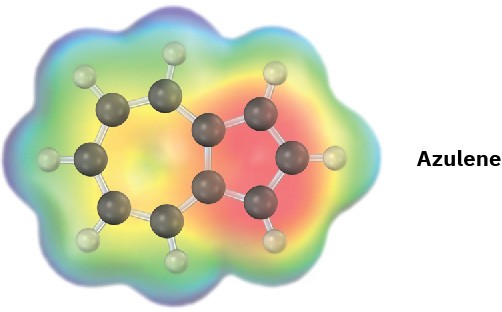
Problem 8.17
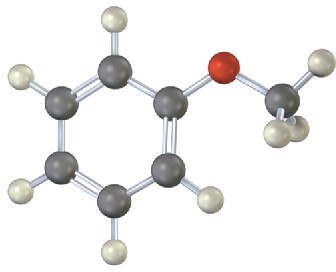
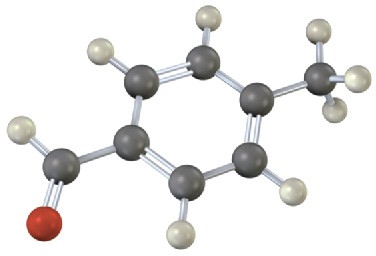
Draw the product from reaction of each of the following substances with (1) Br2, FeBr3 and (2) CH3COCl, AlCl3.
(a)
(b)
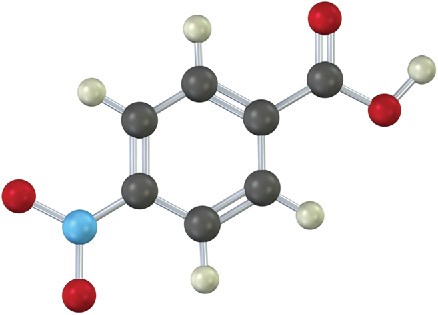
Problem 8.18
How would you synthesize the following compound starting from benzene? More than one step is needed.
Problem 8.19
The following compound can’t be synthesized using the methods discussed in this chapter. Why not?
Naming Aromatic Compounds
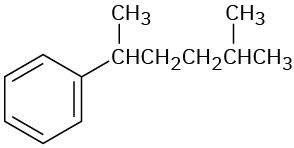
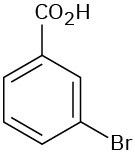
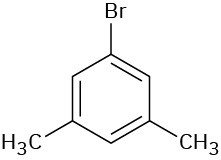
Problem 8.20
Give IUPAC names for the following compounds:
(a)
(b)
(c)
(d)
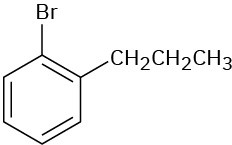
(e)
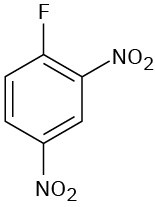
(f)
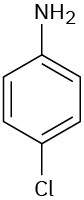
Problem 8.21
Draw structures corresponding to the following names:
(a) 3-Methyl-1,2-benzenediamine
(b) 1,3,5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2,4,6-Trinitrophenol (picric acid)
Problem 8.22
Draw and name all possible isomers of the following:
(a) Dinitrobenzene
(b) Bromodimethylbenzene
(c) Trinitrophenol
Problem 8.23
Draw and name all possible aromatic compounds with the formula C7H7Cl.
Problem 8.24
Draw and name all possible aromatic compounds with the formula C8H9Br. (There are 14.)
Structure of Aromatic Compounds
Problem 8.25
Propose structures for aromatic hydrocarbons that meet the following descriptions:
(a) C9H12; gives only one C9H11Br product on substitution of a hydrogen on the aromatic ring with bromine
(b) C10H14; gives only one C10H13Cl product on substitution of a hydrogen on the aromatic ring with chlorine
(c) C8H10; gives three C8H9Br products on substitution of a hydrogen on the aromatic ring with bromine
(d) C10H14; gives two C10H13Cl products on substitution of a hydrogen on the aromatic ring with chlorine
Problem 8.26
Look at the three resonance structures of naphthalene shown in Section 8.9, and account for the fact that not all carbon–carbon bonds have the same length. The C1–C2 bond is 136 pm long, whereas the C2–C3 bond is 139 pm long.
Problem 8.27
Anthracene has four resonance structures, one of which is shown. Draw the other three.
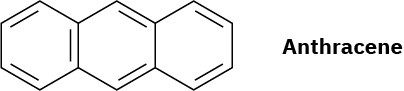
Problem 8.28
Phenanthrene has five resonance structures, one of which is shown. Draw the other four.
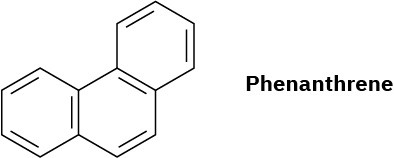
Problem 8.29
Look at the five resonance structures for phenanthrene (Problem 8.28), and predict which of its carbon–carbon bonds is shortest.
Problem 8.30
Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an orbital picture of indole.
(a) How many π electrons does indole have?
(b) What is the electronic relationship of indole to naphthalene?
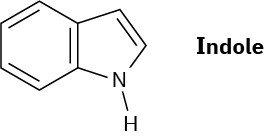
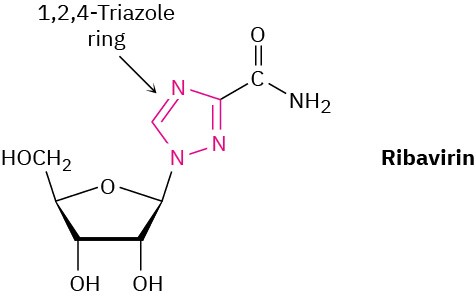
Problem 8.31
Ribavirin, an antiviral agent used against hepatitis C and viral pneumonia, contains a 1,2,4- triazole ring. Why is the ring aromatic?
Mechanisms of Electrophilic Substitutions
Problem 8.32
Aromatic iodination can be carried out with a number of reagents, including iodine monochloride, ICl. What is the direction of polarization of ICl? Propose a mechanism for the iodination of an aromatic ring with ICl.
Problem 8.33
The N,N,N-trimethylammonium group, –N(CH3)3, is one of the few groups that is a meta-directing deactivator yet has no electron-withdrawing resonance effect. Explain.
Problem 8.34
Using resonance structures of the intermediates, explain why bromination of biphenyl occurs at ortho and para positions rather than at meta.
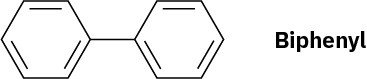
Problem 8.35
Addition of HBr to 1-phenylpropene yields only (1-bromopropyl)benzene. Propose a mechanism for the reaction, and explain why none of the other regioisomer is produced.
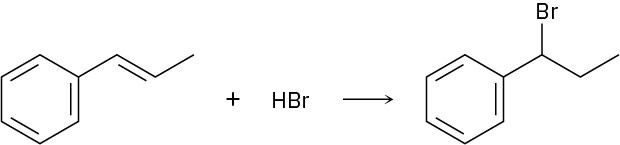
Problem 8.36
Propose a mechanism to account for the following reaction:
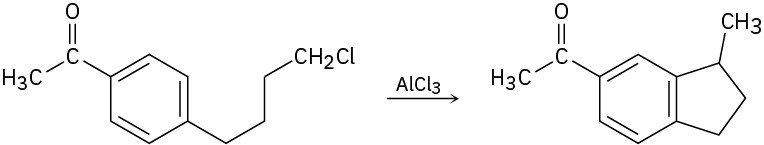
Problem 8.37
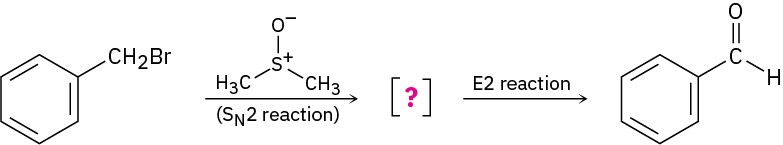
Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in the reaction.
Reactivity and Orientation of Electrophilic Substitutions
Problem 8.38
Identify each of the following groups as an activator or deactivator and as an o,p-director or m-director:
(a)
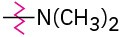
(b)
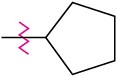
(c)
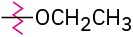
(d)
Problem 8.39
Predict the major product(s) of nitration of the following substances. Which react faster than benzene, and which slower?
(a) Bromobenzene
(b) Benzonitrile
(c) Benzoic acid
(d) Nitrobenzene
(e) Benzenesulfonic acid
(f) Anisole (methoxybenzene)
Problem 8.40
Rank the compounds in each group according to their reactivity toward electrophilic substitution.
(a) Chlorobenzene, o-dichlorobenzene, benzene
(b) p-Bromonitrobenzene, nitrobenzene, phenol
(c) Fluorobenzene, benzaldehyde, o-xylene
(d) Benzonitrile, p-methylbenzonitrile, p-methoxybenzonitrile
Problem 8.41
Predict the major monoalkylation products you would expect to obtain from reaction of the following substances with chloromethane and AlCl3:
(a) Bromobenzene
(b) m-Bromophenol
(c) p-Chloroaniline
(d) 2,4-Dichloronitrobenzene
(e) 2,4-Dichlorophenol
(f) Benzoic acid
(g) p-Methylbenzenesulfonic acid
(h) 2,5-Dibromotoluene
Problem 8.42
Rank the following aromatic compounds in the expected order of their reactivity toward Friedel–Crafts alkylation. Which compounds are unreactive?
(a) Bromobenzene
(b) Toluene
(c) Phenol
(d) Aniline
(e) Nitrobenzene
(f) p-Bromotoluene
Problem 8.43
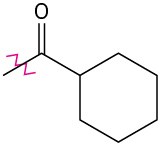
What product(s) would you expect to obtain from the following reaction?
(a)
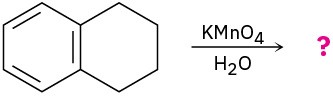
Problem 8.44
Predict the major product(s) of the following reactions:
(a)
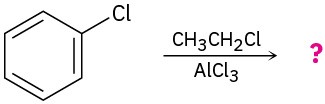
(b)
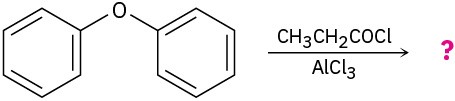
(c)
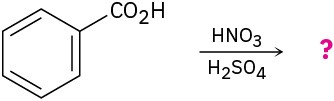
(d)
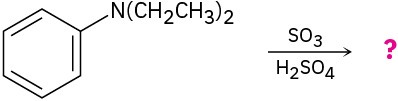
Organic Synthesis
Problem 8.45
How would you synthesize the following substances starting from benzene or phenol? Assume that ortho- and para-substitution products can be separated.
(a) o-Bromobenzoic acid
(b) p-Methoxytoluene
(c) m-Bromoaniline
Problem 8.46
Starting with benzene as your only source of aromatic compounds, how would you synthesize the following substances? Assume that you can separate ortho and para isomers if necessary.
(a) p-Chloroacetophenone
(b) m-Bromonitrobenzene
(c) o-Bromobenzenesulfonic acid
(d) m-Chlorobenzenesulfonic acid
General Problems
Problem 8.47
Aromatic substitution reactions occur by addition of an electrophile such as Br+ to an aromatic ring to yield an allylic carbocation intermediate, followed by loss of H+. Show the structure of the intermediate formed by reaction of benzene with Br+.
Problem 8.48
The substitution reaction of toluene with Br2 can, in principle, lead to the formation of three isomeric bromotoluene products. In practice, however, only o– and p-bromotoluene are formed in substantial amounts. The meta isomer is not formed. Draw the structures of the three possible carbocation intermediates and explain why ortho and para products predominate over meta products.
Problem 8.49
Look at the following aromatic anions and their linear counterparts, and draw all of the resonance forms for each. What patterns emerge?
(a)
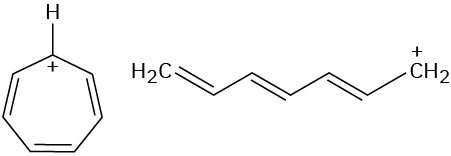
(b)
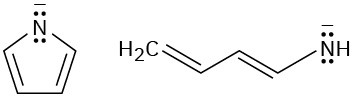
Problem 8.50
Compounds called azo dyes are the major source of artificial color in textiles and food. Part of the reason for their intense coloring is the conjugation from an electron-donating group through the diazo bridge (−N=N−) to an electron-withdrawing group on the other side. For the following azo dyes, draw a resonance form that shows how the electron-donating group is related to the electron-withdrawing group on the other side of the diazo bridge. Used curved arrows to show how the electrons are reorganized.
(a)
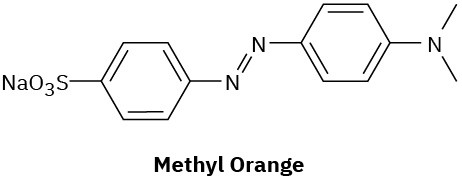
(b)
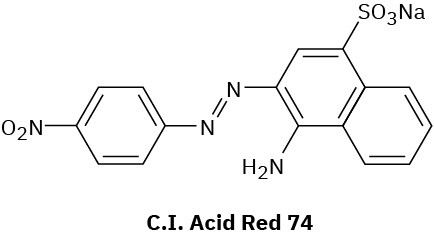
Problem 8.51
Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2.
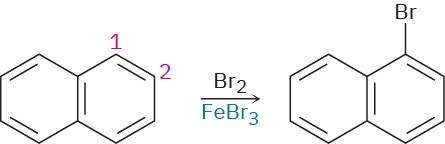
Problem 8.52
How would you synthesize the following compounds from benzene? Assume that ortho and para isomers can be separated.
(a)
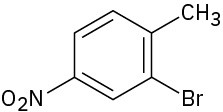
Problem 8.53
You know the mechanism of HBr addition to alkenes, and you know the effects of various substituent groups on aromatic substitution. Use this knowledge to predict which of the following two alkenes reacts faster with HBr. Explain your answer by drawing resonance structures of the carbocation intermediates.
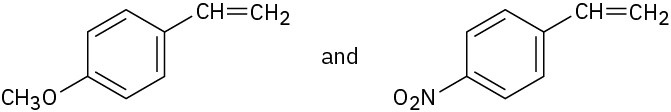
Problem 8.54
Phenols (ArOH) are relatively acidic, and the presence of a substituent group on the aromatic ring has a large effect. The pKa of unsubstituted phenol, for example, is 9.89, while that of p-nitrophenol is 7.15. Draw resonance structures of the corresponding phenoxide anions and explain the data.
Problem 8.55
Would you expect p-methylphenol to be more acidic or less acidic than unsubstituted phenol? Explain. (See Problem 8.54)
Problem 8.56
Predict the product(s) for each of the following reactions. In each case, draw the resonance forms of the intermediate to explain the observed regiochemistry.
(a)
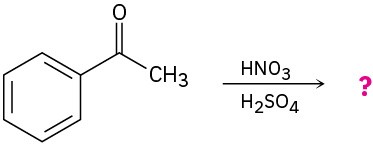
(b)
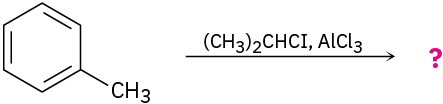
(c)
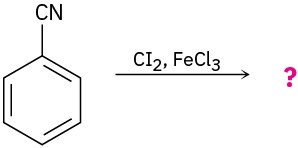
(d)