Additional Problems 9
Visualizing Chemistry
Problem 9.23
Give IUPAC names for the following compounds:
(a)
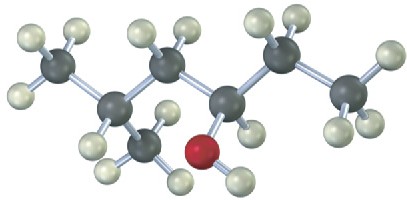
(b)
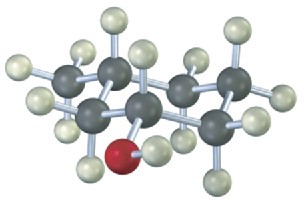
(c)
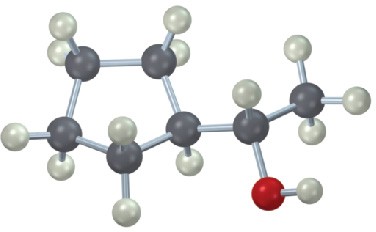
(d)
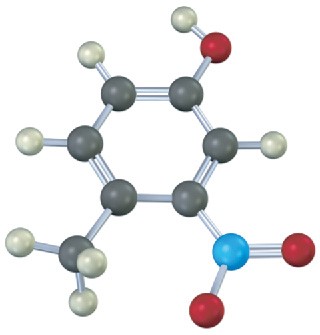
Problem 9.24
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (1) Na metal, (2) SOCl2, and (3) Dess–Martin periodinane.
(a)
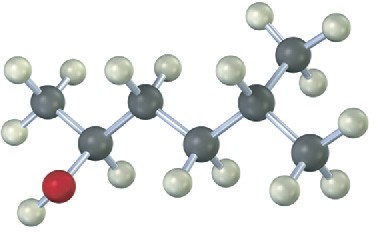
(b)
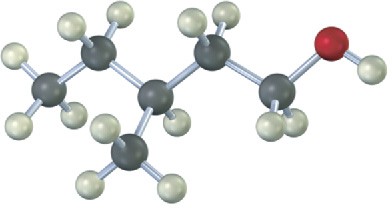
Problem 9.25
Predict the product from reaction of the following substance (reddish brown = Br) with:
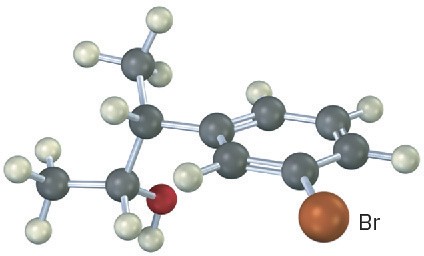
(a) PBr3
(b) Aqueous H2SO4
(c) SOCl2
(d) Dess–Martin periodinane
Problem 9.26
Predict the product from reaction of the following substance with:
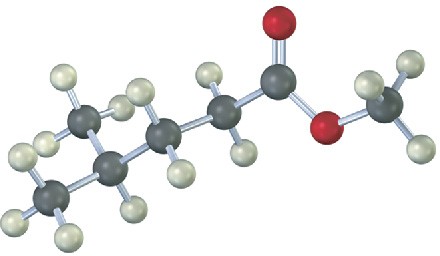
(a) NaBH4; then H3O+
(b) 2 CH3CH2MgBr; then H3O+
Problem 9.27
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethylmagnesium bromide. Is the product chiral? Is it optically active? Explain.
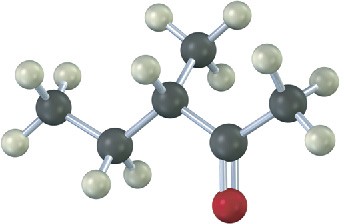
Problem 9.28
Give IUPAC names for the following compounds (red = O; reddish brown = Br; yellow = S):
(a)
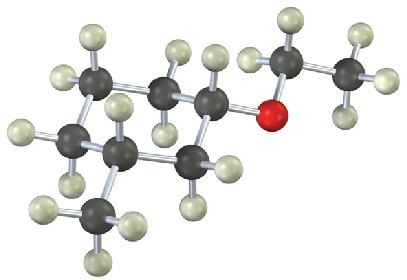
(b)
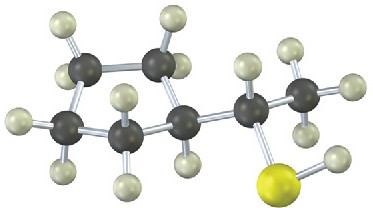
Problem 9.29
Show the product, including stereochemistry, of the following reaction:
Mechanism Problems
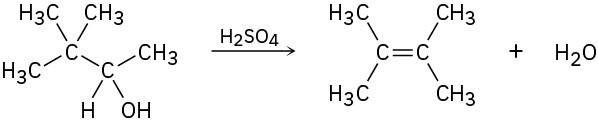
Problem 9.30
Evidence for the intermediate carbocations in the acid-catalyzed dehydration of alcohols comes from the observation that rearrangements sometimes occur. Propose a mechanism to account for the formation of 2,3-dimethyl-2-butene from 3,3-dimethyl-2-butanol.
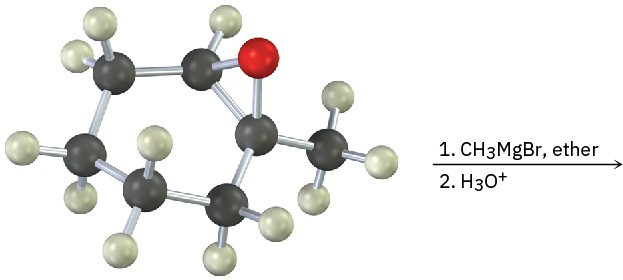
Problem 9.31
Epoxides react with Grignard reagents to yield alcohols. Propose a mechanism.
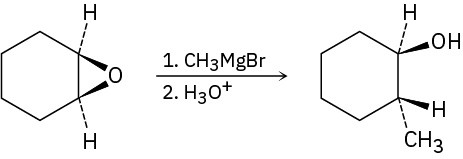
Problem 9.32
Reduction of 2-butanone with NaBH4 yields 2-butanol. Is the product chiral? Is it optically active? Explain.
Problem 9.33
The conversion of 3° alcohols into 3° alkyl halides under acidic conditions involves two cationic intermediates. For each reaction, draw the complete mechanism using curved arrows.
(a)
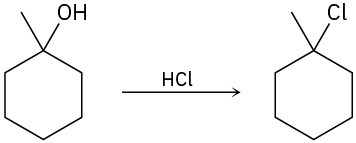
(b)
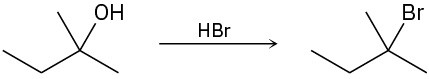
(c)
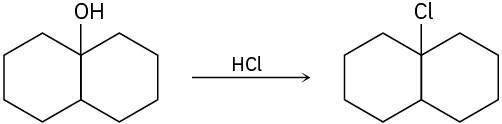
Problem 9.34
Identify the type of substitution mechanism (SN1, SN2) involved in the conversion of the following alcohols into the corresponding alkyl halide.
(a)
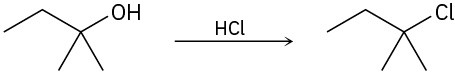
(b)
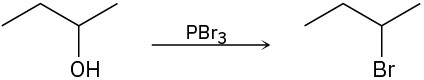
Problem 9.35
The conversion of 3° alcohols into alkenes under acidic conditions involves two cationic intermediates. For each reaction, draw the complete mechanism using curved arrows.
(a)
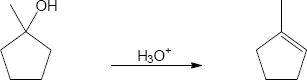
(b)
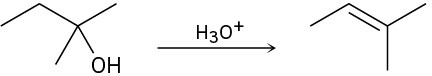
(c)
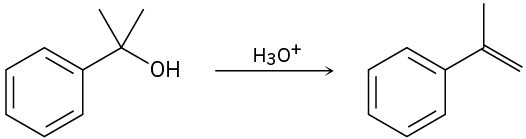
Problem 9.36
Phenols generally have lower pKa’s than alcohols because of resonance stabilization with the aromatic ring. Draw all of the resonance contributors for the following phenolate ions.
(a)
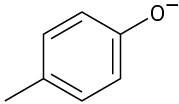
(b)
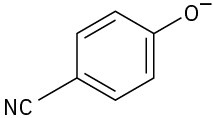
(c)
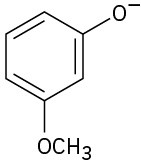
Problem 9.37
Predict the product(s) and provide the mechanism for each of the following reactions.
(a)
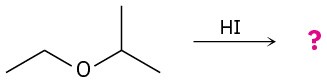
(b)
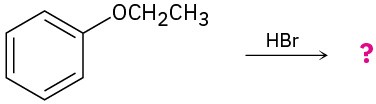
Problem 9.38
Predict the product(s) and provide the mechanism for each of the following reactions.
(a)
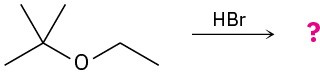
(b)
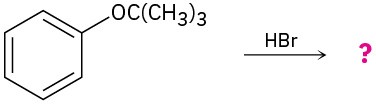
Problem 9.39
Predict the product(s) and provide the mechanism for the following two-step processes.
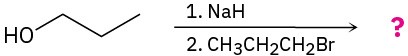
Problem 9.40
Predict the product(s) and provide the mechanism for the following reaction:
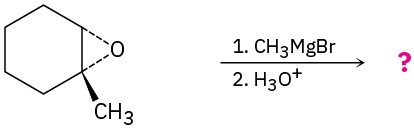
Problem 9.41
Fluoxetine, a heavily prescribed antidepressant marketed under the name Prozac, can be prepared by a route that begins with reaction between a phenol and an alkyl chloride.
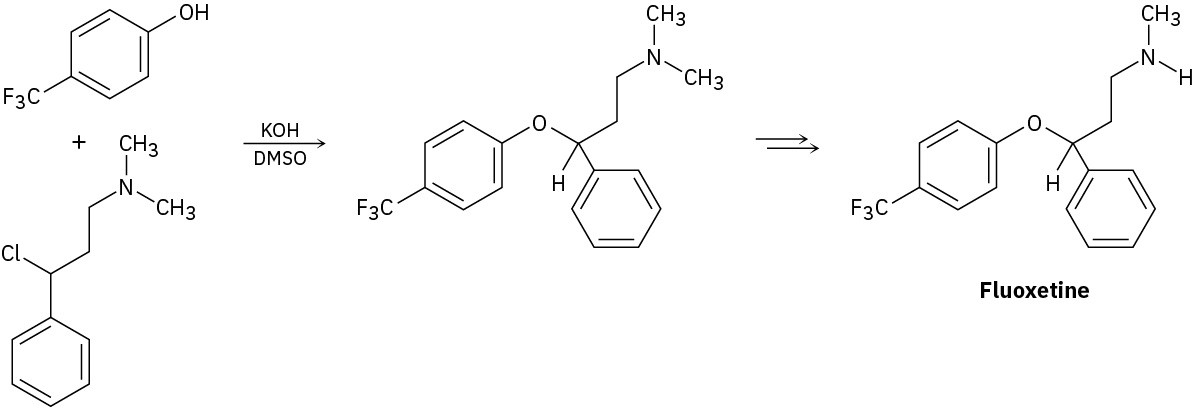
(a) The rate of the reaction depends on both phenol and alkyl halide. Is this an SN1 or an SN2 reaction? Show the mechanism.
(b) The physiologically active enantiomer of fluoxetine has (S) stereochemistry. Based on your answer in part (a), draw the structure of the alkyl chloride you would need, showing the correct stereochemistry.
Problem 9.42
The herbicide acifluorfen can be prepared by a route that begins with reaction between a phenol and an aryl fluoride. Propose a mechanism.
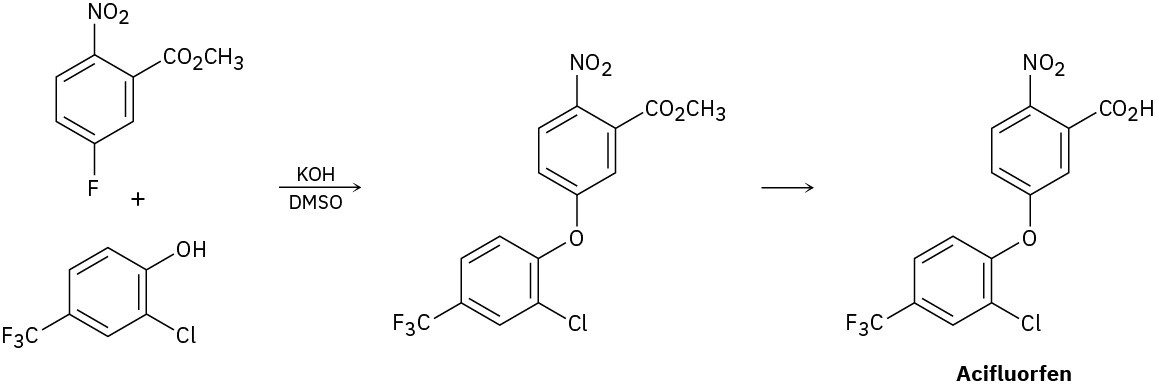
Problem 9.43
Aldehydes and ketones undergo acid-catalyzed reaction with alcohols to yield hemiacetals, from aldehydes or ketals with ketones compounds that have one alcohol-like oxygen and one ether-like oxygen bonded to the same carbon. Further reaction of a hemiacetal with alcohol then yields an acetal, a compound that has two ether-like oxygens bonded to the same carbon.
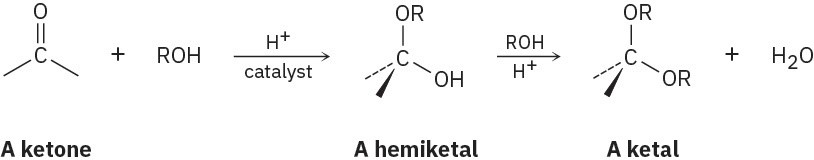
(a) Show the structures of the hemiketal and ketal you would obtain by reaction of cyclohexanone with ethanol.
(b) Propose a mechanism for the conversion of a hemiacetal into a ketal.
Naming Alcohols
Problem 9.44
Give IUPAC names for the following compounds:
(a)
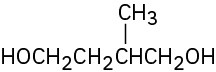
(b)
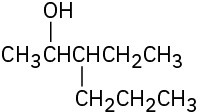
(c)
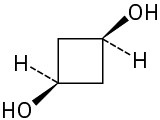
(d)
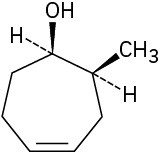
(e)
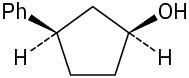
(f)
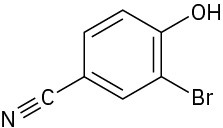
Problem 9.45
Draw and name the eight isomeric alcohols with formula C5H12O.
Problem 9.46
Draw structures corresponding to the following IUPAC names:
(a) Trans-3-Chlorocycloheptanol
(b) Ethyl-2-buten-1-ol
(c) o-(2-Hydroxyethyl)phenol
(d) Methyl-1-phenyl-1-butanol
Problem 9.47
Bombykol, the sex pheromone secreted by the female silkworm moth has the formula C16H28O and the systematic name (10E,12Z)-10,12-hexadecadien-1-ol. Draw bombykol, showing the correct geometry for the two double bonds.
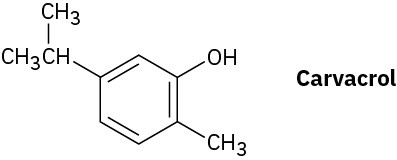
Problem 9.48
Carvacrol is a naturally occurring substance isolated from oregano, thyme, and marjoram. What is its IUPAC name?
Naming Ethers, Thiols and Sulfides
Problem 9.49
Draw structures corresponding to the following IUPAC names:
(a) Ethyl 1-ethylpropyl ether
(b) Di(p-chlorophenyl) ether
(c) 3,4-Dimethoxybenzoic acid
(d) Cyclopentyloxycyclohexane
Problem 9.50
Give IUPAC names for the following structures:
(a)
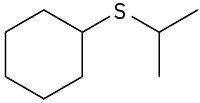
(b)
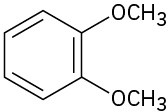
(c)
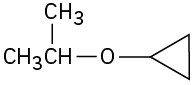
(d)
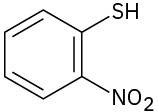
(e)
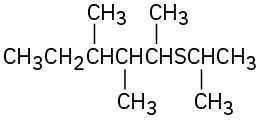
(f)
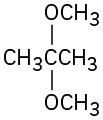
(g)
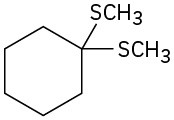
Synthesizing Alcohols
Problem 9.51
What Grignard reagent and what carbonyl compound might you start with to prepare the following alcohols:
(a)
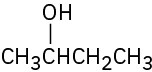
(b)
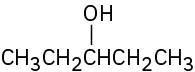
(c)
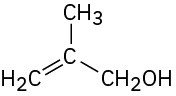
(d)
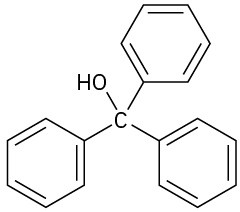
(e)
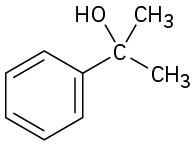
(f)
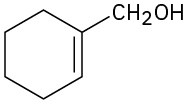
Problem 9.52
What carbonyl compounds would you reduce to prepare the following alcohols: List all possibilities.
(a)
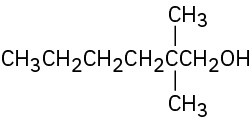
(b)
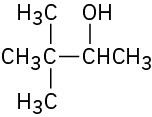
(c)
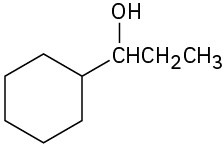
Problem 9.53
What carbonyl compounds might you start with to prepare the following compounds by Grignard reaction? List all possibilities.
(a) 2-Methyl-2-propanol
(b) 1-Ethylcyclohexanol
(c) 3-Phenyl-3-pentanol
(d) 2-Phenyl-2-pentanol
(e)
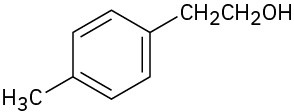
(f)
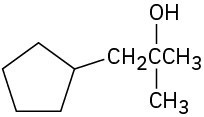
Problem 9.54
How would you synthesize the following alcohols, starting with benzene and other alcohols of six or fewer carbons as your only organic reagents?
(a)
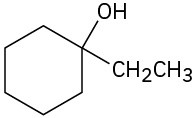
(b)
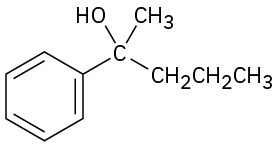
(c)
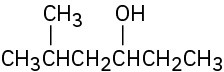
Reactions of Alcohols
Problem 9.55
What products would you obtain from reaction of 1-pentanol with the following reagents:
(a) PBr3
(b) SOCl2
(c) Dess–Martin periodinane
Problem 9.56
How would you prepare the following compounds from 2-phenylethanol: More than one step may be required.
(a) Phenylacetaldehyde (PhCH2CHO)
(b) Phenylacetic acid (PhCH2CO2H)
(c) Ethylbenzene
(d) Bromo-2-phenylethane
Problem 9.57
How would you prepare the following compound from 1-phenylethanol: More than one step may be required.
Acetophenone (PhCOCH3)
Problem 9.58
How would you prepare the following substances from cyclopentanol: More than one step may be required.
(a) Cyclopentanone
(b) Cyclopentene
Problem 9.59
What products would you expect to obtain from reaction of 1-methylcyclohexanol with the following reagents?
(a) NaH
(b) HBr
(c) H2SO4
Synthesizing Ethers
Problem 9.60
How would you prepare the following ethers?
(a)
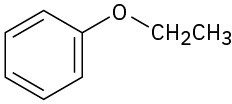
(b)
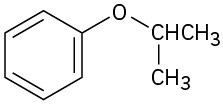
(c)
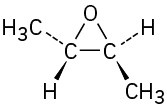
(d)
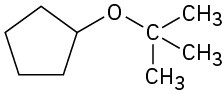
(e)
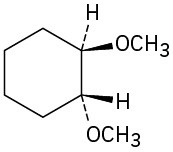
Problem 9.61
How would you prepare the following compounds from 1-phenylethanol?
(a) Methyl 1-phenylethyl ether
(b) tert-Butyl 1-phenylethyl ether
Problem 9.62
Treatment of trans-2-chlorocyclohexanol with NaOH yields 1,2-epoxycyclohexane, but reaction of the cis isomer under the same conditions yields cyclohexanone. Propose mechanisms for both reactions, and explain why the different results are obtained.
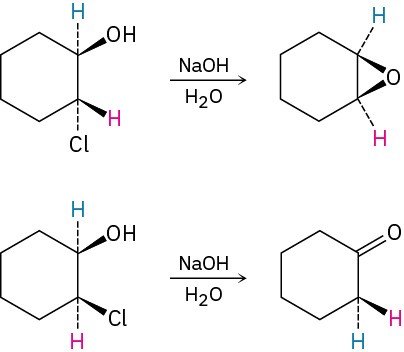
Reactions of Ethers and Epoxides
Problem 9.63
Predict the products of the following ether cleavage reactions:
(a)
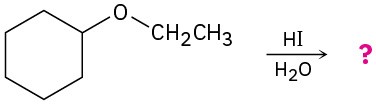
(b)
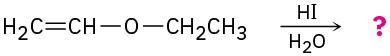
(c)
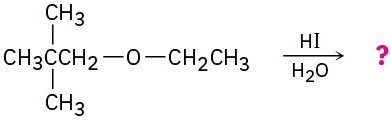
Problem 9.64
How would you carry out the following transformation? More than one step may be required.
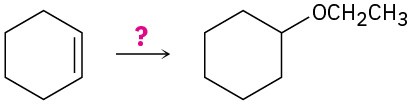
Problem 9.65
What product would you expect from cleavage of tetrahydrofuran with HI?
Problem 9.66
Write the mechanism of the hydrolysis of cis-5,6-epoxydecane by reaction with aqueous acid. What is the stereochemistry of the product, assuming normal backside SN2 attack?
Problem 9.67
What is the stereochemistry of the product from acid-catalyzed hydrolysis of trans-5,6- epoxydecane? How does the product differ from that formed in Problem 18-47?
Problem 9.68
Epoxides are reduced by treatment with lithium aluminum hydride to yield alcohols. Propose a mechanism for this reaction.
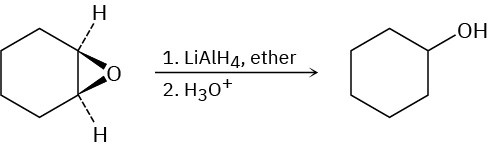
General Problems
Problem 9.69
How would you carry out the following transformations?
(a)

(b)
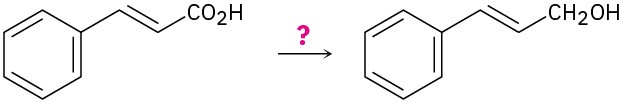
Problem 9.70
Rank the following substituted phenols in order of increasing acidity, and explain your answer:
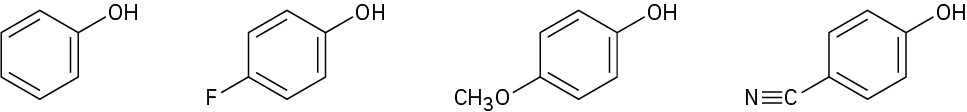
Problem 9.71
Reaction of (S)-3-methyl-2-pentanone with methylmagnesium bromide followed by acidification yields 2,3-dimethyl-2-pentanol. What is the stereochemistry of the product? Is the product optically active?

Problem 9.72
As a rule, axial alcohols oxidize somewhat faster than equatorial alcohols. Which would you expect to oxidize faster, cis-4-tert-butylcyclohexanol or trans-4-tert-butylcyclohexanol? Draw the more stable chair conformation of each molecule.
Problem 9.73
A problem often encountered in the oxidation of primary alcohols to carboxylic acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate:
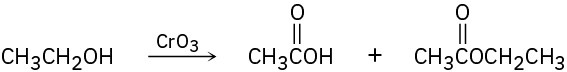
Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:
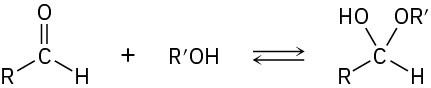
Problem 9.74
Identify the reagents a–f in the following scheme:
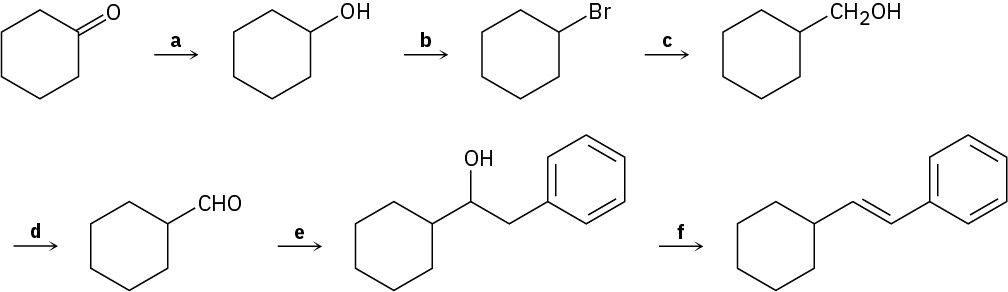
Problem 9.75
Predict the products of the following reactions:
(a)
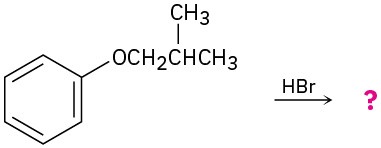
Problem 9.76
Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene oxide. Suggest a reason for the difference in reactivity between oxetane and ethylene oxide.
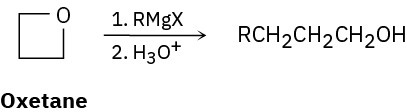
Problem 9.77
The Zeisel method is an old analytical procedure for determining the number of methoxyl groups in a compound. A weighed amount of the compound is heated with concentrated HI, ether cleavage occurs, and the iodomethane product is distilled off and passed into an alcohol solution of AgNO3, where it reacts to form a precipitate of silver iodide. The AgI is then collected and weighed, and the percentage of methoxyl groups in the sample is thereby determined. For example, 1.06 g of vanillin, the material responsible for the characteristic odor of vanilla, yields 1.60 g of AgI. If vanillin has a molecular weight of 152, how many methoxyl groups does it contain?
Problem 9.78
Identify the reagents a–e in the following scheme: