1.3 Atomic Structure: Electron Configurations
The lowest-energy arrangement, or ground-state electron configuration, of an atom is a list of the orbitals occupied by its electrons. We can predict this arrangement by following three rules.
RULE 1
The lowest-energy orbitals fill up first, 1𝑠 → 2𝑠 → 2𝑝 → 3𝑠 → 3𝑝 → 4𝑠 → 3𝑑, according to the following graphic, a statement called the Aufbau principle. Note that the 4s orbital lies between the 3p and 3d orbitals in energy.
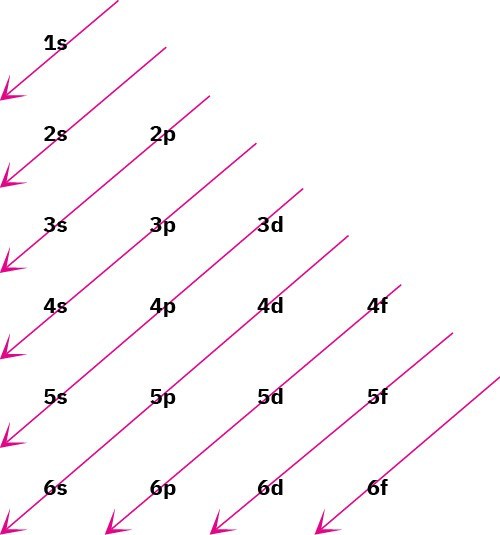
RULE 2
Electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. This spin can have two orientations, denoted as up (↑) and down (↓). Only two electrons can occupy an orbital, and they must have opposite spins, a statement called the Pauli exclusion principle.
RULE 3
If two or more empty orbitals of equal energy are available, one electron occupies each with spins parallel until all orbitals are half-full, a statement called Hund’s rule.
Some examples of how these rules apply are shown in Table 1.1. Hydrogen, for instance, has only one electron, which must occupy the lowest-energy orbital. Thus, hydrogen has a 1s ground-state configuration. Carbon has six electrons and the ground-state configuration 1s22s22px12py1, and so forth. Note that a superscript is used to represent the number of electrons in a particular orbital.
Table 1.1 Ground-State Electron Configurations of Some Elements
|
Element |
Atomic number |
Configuration |
|
Hydrogen |
1 |
|
|
Carbon |
6 |
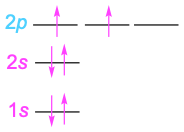 |
|
Phosphorus |
15 |
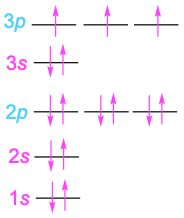 |
Problem 1.1
(a) Oxygen
(b) Nitrogen
(c) Sulfur
Problem 1.2
How many electrons does each of the following biological trace elements have in its outermost electron shell?
(a) Magnesium
(b) Cobalt
(c) Selenium

