10.10 Biological Reductions
As a general rule, nucleophilic addition reactions are characteristic only of aldehydes and ketones, not of carboxylic acid derivatives. The reason for the difference is structural. As discussed previously in the Preview of Carbonyl Chemistry, and shown in Figure 10.9, the tetrahedral intermediate produced by addition of a nucleophile to a carboxylic acid derivative can eliminate a leaving group, leading to a net nucleophilic acyl substitution reaction. The tetrahedral intermediate produced by addition of a nucleophile to an aldehyde or ketone, however, has only alkyl or hydrogen substituents and thus can’t usually expel a leaving group. One exception to this rule is the Cannizzaro reaction, discovered in 1853.
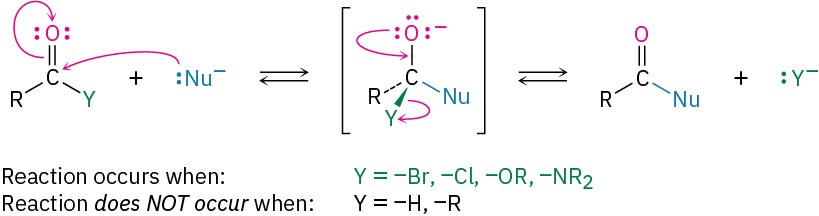 Figure 10.9 Carboxylic acid derivatives have an electronegative substituent Y = – Br, –Cl, –OR, –NR2 that can be expelled as a leaving group from the tetrahedral intermediate formed by nucleophilic addition. Aldehydes and ketones have no such leaving group and thus do not usually undergo this reaction.
Figure 10.9 Carboxylic acid derivatives have an electronegative substituent Y = – Br, –Cl, –OR, –NR2 that can be expelled as a leaving group from the tetrahedral intermediate formed by nucleophilic addition. Aldehydes and ketones have no such leaving group and thus do not usually undergo this reaction.
The Cannizzaro reaction takes place by nucleophilic addition of OH– to an aldehyde to give a tetrahedral intermediate, which expels hydride ion as a leaving group and is thereby oxidized. A second aldehyde molecule accepts the hydride ion in another nucleophilic addition step and is thereby reduced. Benzaldehyde, for instance, yields benzyl alcohol plus benzoic acid when heated with aqueous NaOH.
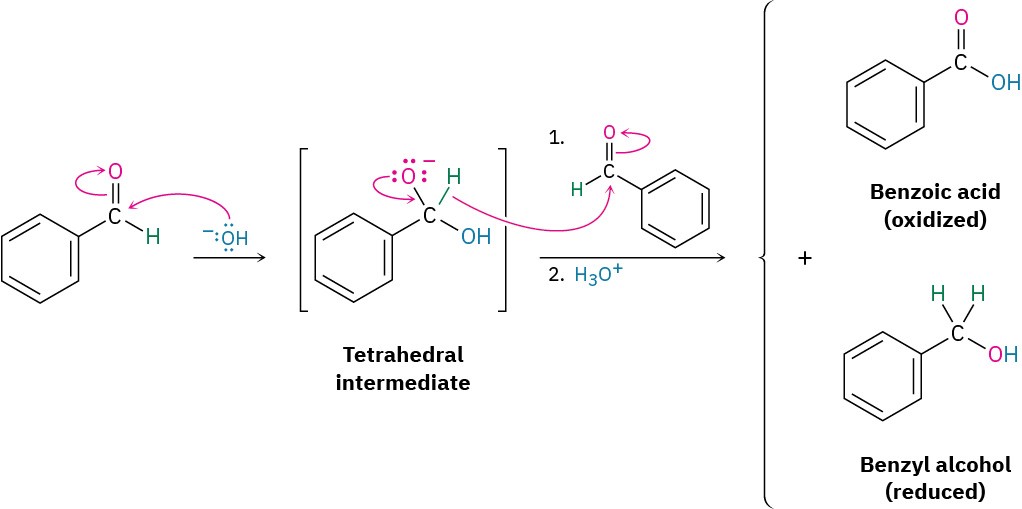 The Cannizzaro reaction is rarely used today but is interesting mechanistically because it is a simple laboratory analogy for the primary biological pathway by which carbonyl reductions occur in living organisms. In nature, as we saw in Section 9.5, one of the most important reducing agents is NADH, reduced nicotinamide adenine dinucleotide. NADH donates H– to aldehydes and ketones, thereby reducing them, in much the same way that the tetrahedral alkoxide intermediate in a Cannizzaro reaction does. The electron lone pair on a nitrogen atom of NADH expels H– as leaving group, which adds to a carbonyl group in another molecule (Figure 10.10). As an example, pyruvate is converted during intense muscle activity to (S)-lactate, a reaction catalyzed by lactate dehydrogenase.
The Cannizzaro reaction is rarely used today but is interesting mechanistically because it is a simple laboratory analogy for the primary biological pathway by which carbonyl reductions occur in living organisms. In nature, as we saw in Section 9.5, one of the most important reducing agents is NADH, reduced nicotinamide adenine dinucleotide. NADH donates H– to aldehydes and ketones, thereby reducing them, in much the same way that the tetrahedral alkoxide intermediate in a Cannizzaro reaction does. The electron lone pair on a nitrogen atom of NADH expels H– as leaving group, which adds to a carbonyl group in another molecule (Figure 10.10). As an example, pyruvate is converted during intense muscle activity to (S)-lactate, a reaction catalyzed by lactate dehydrogenase.
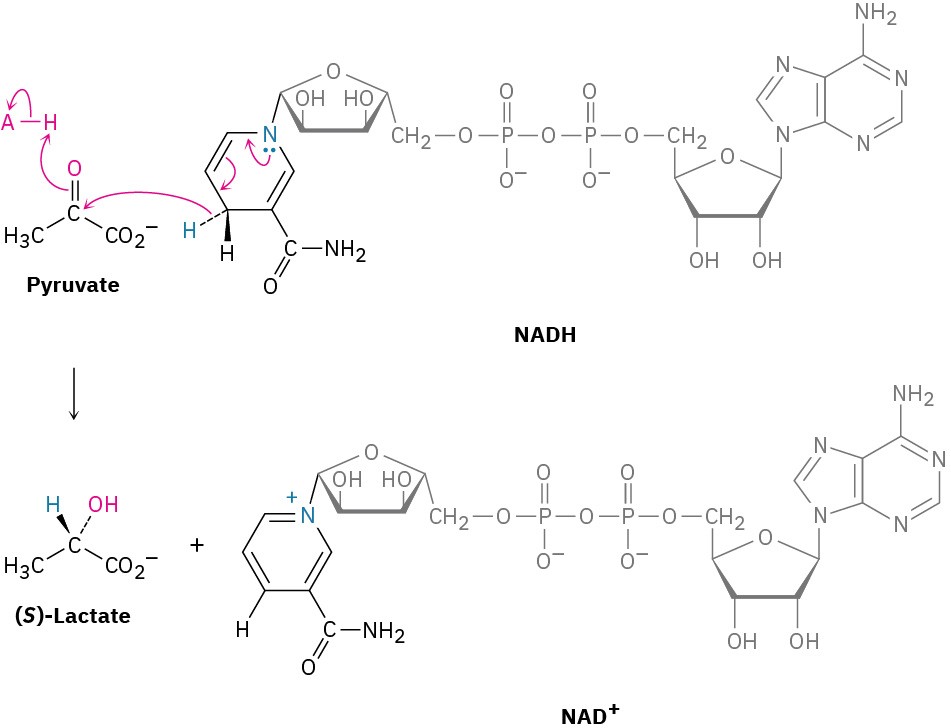 Figure 10.10 Mechanism of biological aldehyde and ketone reductions by the coenzyme NADH. The key step is an expulsion of hydride ion from NADH and donation to the carbonyl group.
Figure 10.10 Mechanism of biological aldehyde and ketone reductions by the coenzyme NADH. The key step is an expulsion of hydride ion from NADH and donation to the carbonyl group.

