11.3 Acidity of Carboxylic Acids
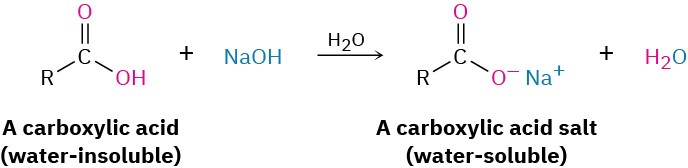
The most obvious property of carboxylic acids is implied by their name: carboxylic acids are acidic. They therefore react with bases such as NaOH and NaHCO3 to give metal carboxylate salts, RCO2− M+. Carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are often highly water- soluble. In fact, it’s often possible to purify an acid by extracting its salt into aqueous base, then reacidifying and extracting the pure acid back into an organic solvent.
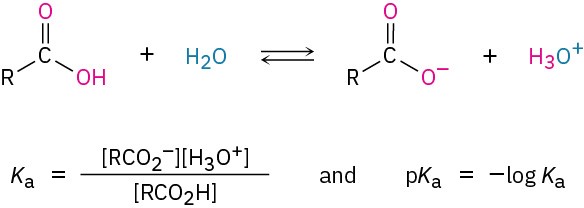
 Like other Brønsted–Lowry acids discussed in Section 1.17, carboxylic acids dissociate slightly in dilute aqueous solution to give H3O+ and the corresponding carboxylate anions, RCO2–. The extent of dissociation is given by an acidity constant, Ka.
Like other Brønsted–Lowry acids discussed in Section 1.17, carboxylic acids dissociate slightly in dilute aqueous solution to give H3O+ and the corresponding carboxylate anions, RCO2–. The extent of dissociation is given by an acidity constant, Ka.
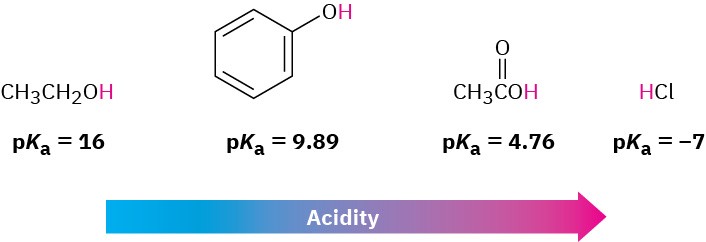
 A list of Ka values for various carboxylic acids is given in Table 11.4. For most, Ka is approximately 10–4 to 10–5. Acetic acid, for instance, has Ka = 1.75 × 10–5 at 25 °C, which corresponds to a pKa of 4.76. In practical terms, a Ka value near 10–5 means that only about 0.1% of the molecules in a 0.1 M solution are dissociated, as opposed to the 100% dissociation found with strong mineral acids like HCl.
A list of Ka values for various carboxylic acids is given in Table 11.4. For most, Ka is approximately 10–4 to 10–5. Acetic acid, for instance, has Ka = 1.75 × 10–5 at 25 °C, which corresponds to a pKa of 4.76. In practical terms, a Ka value near 10–5 means that only about 0.1% of the molecules in a 0.1 M solution are dissociated, as opposed to the 100% dissociation found with strong mineral acids like HCl.
Table 11.4 Acidity of Some Carboxylic Acids
|
Structure |
Ka |
pKa |
|
| CF3CO2H | 0.59 |
0.23 |
|
| HCO2H | 1.77 × 10-4 |
3.75 |
|
| HOCH2CO2H | 1.5 × 10-4 |
3.84 |
|
| C6H5CO2H | 6.46 × 10-5 |
4.19 |
|
| H2C=CHCO2H | 5.6 × 10-5 |
4.25 |
|
| CH3CO2H | 1.75 × 10-5 |
4.76 |
|
| CH3CH2CO2H | 1.34 × 10-5 |
4.87 |
|
| CH3CH2OH (ethanol) | 1 × 10-16 |
16 |
Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. The Ka of ethanol, for example, is approximately 10–16, making it a weaker acid than acetic acid by a factor of 1011.
Why are carboxylic acids so much more acidic than alcohols, even though both contain –OH groups? An alcohol dissociates to give an alkoxide ion, in which the negative charge is localized on a single electronegative atom. A carboxylic acid, however, gives a carboxylate ion, in which the negative charge is delocalized over two equivalent oxygen atoms (Figure 11.2). In resonance terms (Section 1.15), a carboxylate ion is a stabilized resonance hybrid of two equivalent structures. Since a carboxylate ion is more stable than an alkoxide ion, it is lower in energy and more favored in the dissociation equilibrium.
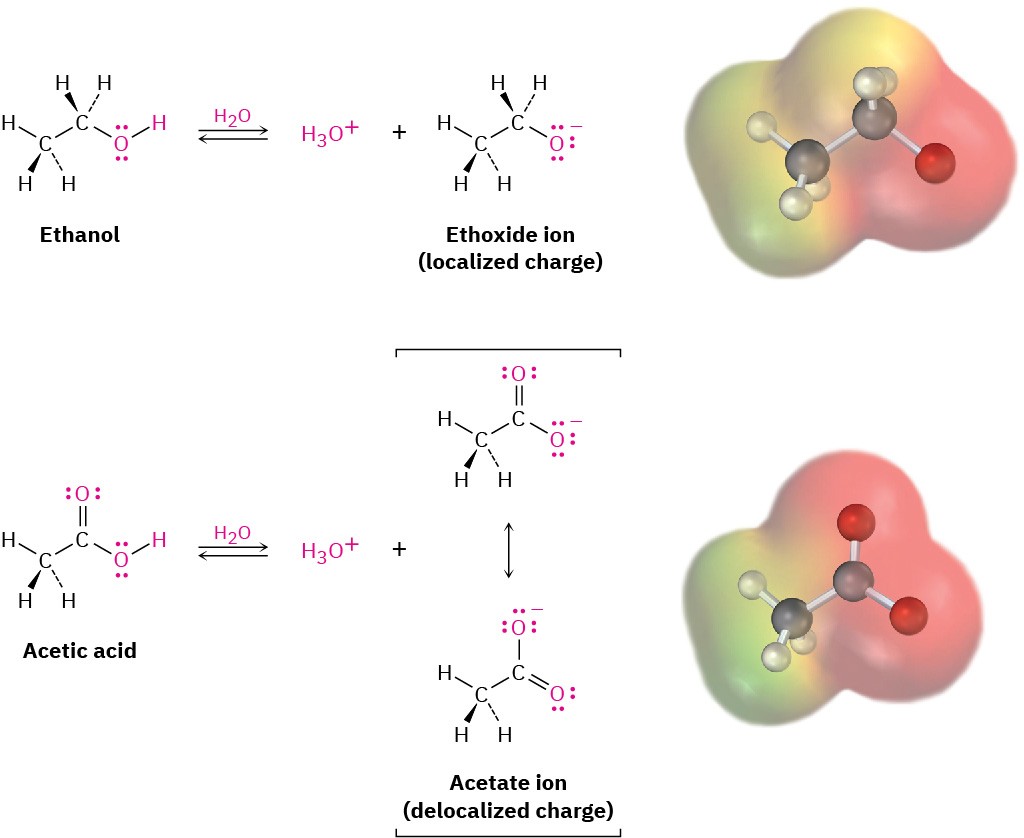
Figure 11.2 An alkoxide ion has its charge localized on one oxygen atom and is less stable, while a carboxylate ion has the charge spread equally over both oxygens and is therefore more stable.
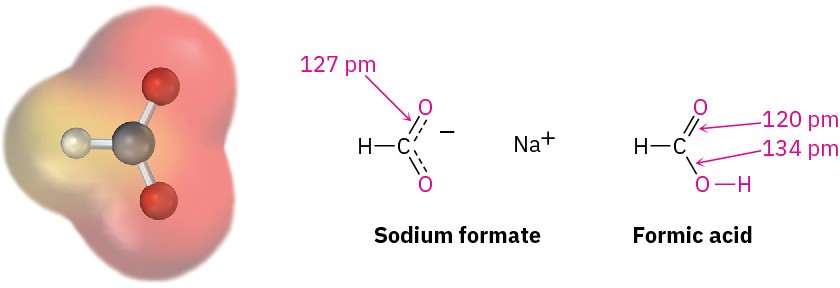
Experimental evidence for the equivalence of the two carboxylate oxygens comes from X-ray crystallographic studies on sodium formate. Both carbon–oxygen bonds are 127 pm in length, midway between the C═O double bond (120 pm) and the C–O single bond (134 pm) of formic acid. An electrostatic potential map of the formate ion also shows how the negative charge (red) is spread equally over both oxygens.
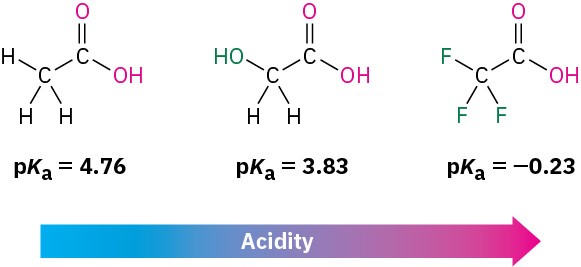
 The listing of pKa values shown previously in Table 11.4 indicates that there are substantial differences in acidity from one carboxylic acid to another. For example, trifluoroacetic acid (Ka = 0.59) is 33,000 times as strong as acetic acid (Ka = 1.75 × 10–5). How can we account for such differences? Because the dissociation of a carboxylic acid is an equilibrium process, any factor that stabilizes the carboxylate anion relative to undissociated carboxylic acid will drive the equilibrium toward increased dissociation and result in increased acidity. For instance, three electron-withdrawing fluorine atoms delocalize the negative charge in the trifluoroacetate anion, thereby stabilizing the ion and increasing the acidity of CF3CO2H. In the same way, glycolic acid (HOCH2CO2H; pKa = 3.83) is stronger than acetic acid because of the electron-withdrawing effect of the electronegative oxygen atom.
The listing of pKa values shown previously in Table 11.4 indicates that there are substantial differences in acidity from one carboxylic acid to another. For example, trifluoroacetic acid (Ka = 0.59) is 33,000 times as strong as acetic acid (Ka = 1.75 × 10–5). How can we account for such differences? Because the dissociation of a carboxylic acid is an equilibrium process, any factor that stabilizes the carboxylate anion relative to undissociated carboxylic acid will drive the equilibrium toward increased dissociation and result in increased acidity. For instance, three electron-withdrawing fluorine atoms delocalize the negative charge in the trifluoroacetate anion, thereby stabilizing the ion and increasing the acidity of CF3CO2H. In the same way, glycolic acid (HOCH2CO2H; pKa = 3.83) is stronger than acetic acid because of the electron-withdrawing effect of the electronegative oxygen atom.
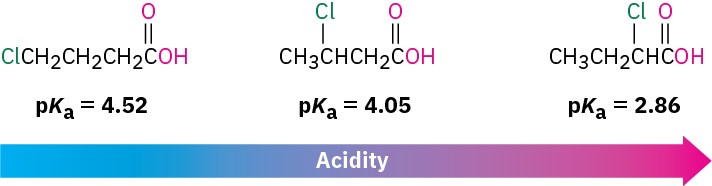
 Because inductive effects operate through σ bonds and are dependent on distance, the effect of halogen substitution decreases as the substituent moves farther from the carboxyl. Thus, 2-chlorobutanoic acid has pKa = 2.86, 3-chlorobutanoic acid has pKa = 4.05, and 4-chlorobutanoic acid has pKa = 4.52, similar to that of butanoic acid itself.
Because inductive effects operate through σ bonds and are dependent on distance, the effect of halogen substitution decreases as the substituent moves farther from the carboxyl. Thus, 2-chlorobutanoic acid has pKa = 2.86, 3-chlorobutanoic acid has pKa = 4.05, and 4-chlorobutanoic acid has pKa = 4.52, similar to that of butanoic acid itself.
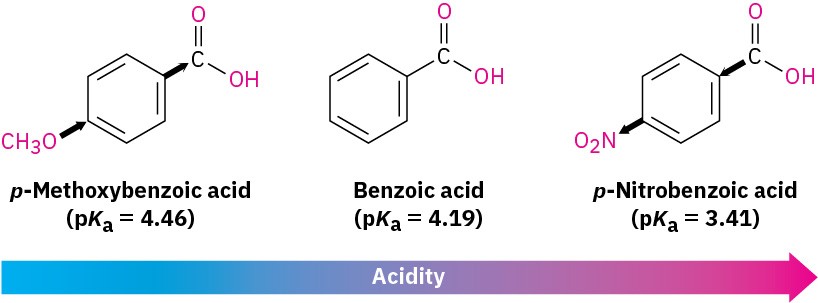
 Substituent effects on acidity are also found in substituted benzoic acids. We said during the discussion of electrophilic aromatic substitution in Sections 8.6 and 8.7 that substituents on the aromatic ring strongly affect reactivity. Aromatic rings with electron-donating groups are activated toward further electrophilic substitution, and aromatic rings with electron-withdrawing groups are deactivated. Exactly the same effects can be observed on the acidity of substituted benzoic acids (Table 11.5).
Substituent effects on acidity are also found in substituted benzoic acids. We said during the discussion of electrophilic aromatic substitution in Sections 8.6 and 8.7 that substituents on the aromatic ring strongly affect reactivity. Aromatic rings with electron-donating groups are activated toward further electrophilic substitution, and aromatic rings with electron-withdrawing groups are deactivated. Exactly the same effects can be observed on the acidity of substituted benzoic acids (Table 11.5).
Table 11.5 Substituent Effects on the Acidity of p-Substituted Benzoic Acids
|
|
Y |
Ka × 10-5 |
pKa |
|
|
|
–NO2 |
39 |
3.41 |
Deactivating groups |
|
–CN |
28 |
3.55 |
||
|
–CHO |
18 |
3.75 |
||
|
–Br |
11 |
3.96 |
||
|
–Cl |
10 |
4.0 |
||
|
–H |
6.46 |
4.19 |
|
|
|
–CH3 |
4.3 |
4.34 |
Activating groups |
|
|
–OCH3 |
3.5 |
4.46 |
||
|
–OH |
3.3 |
4.48 |
As Table 11.5 shows, an electron-donating (activating) group such as methoxy decreases acidity by destabilizing the carboxylate anion, and an electron-withdrawing (deactivating) group such as nitro increases acidity by stabilizing the carboxylate anion.
 Problem 11.5
Problem 11.5
Assume you have a mixture of naphthalene and benzoic acid that you want to separate. How might you take advantage of the acidity of one component in the mixture to effect a separation?
Problem 11.6
Which would you expect to be a stronger acid, the lactic acid found in tired muscles or acetic acid? Explain.
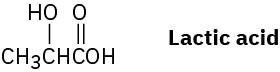 Problem 11.7
Problem 11.7
Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a monoanion and one for the second dissociation into a dianion. For oxalic acid, HO2C–CO2H, the first ionization constant is pKa1 = 1.2 and the second ionization constant is pKa2 = 4.2.
Why is the second carboxyl group far less acidic than the first?
Problem 11.8
Rank the following compounds in order of increasing acidity. Don’t look at a table of pKa data to help with your answer.
(a) Benzoic acid, p-methylbenzoic acid, p-chlorobenzoic acid
(b) p-Nitrobenzoic acid, acetic acid, benzoic acid



