7.5 Characteristics of the SN1 Reaction
Just as the SN2 reaction is strongly influenced by the structure of the substrate, the leaving group, the nucleophile, and the solvent, the SN1 reaction is similarly influenced. Factors that lower ∆G‡, either by lowering the energy level of the transition state or by raising the energy level of the ground state, favor faster SN1 reactions. Conversely, factors that raise
∆G‡, either by raising the energy level of the transition state or by lowering the energy level of the reactant, slow down the SN1 reaction.
The Substrate
Because of resonance stabilization, a primary allylic or benzylic carbocation is about as stable as a secondary alkyl carbocation, and a secondary allylic or benzylic carbocation is about as stable as a tertiary alkyl carbocation. This stability order of carbocations is the same as the order of SN1 reactivity for alkyl halides and tosylates.
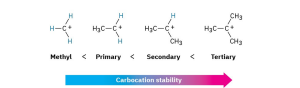 Problem 7.9
Problem 7.9
Rank the following substances in order of their expected SN1 reactivity:
2-bromo-2-methylpropane, 1-bromoethane, 2-bromopropane
The Leaving Group
We said during the discussion of SN2 reactivity that the best leaving groups are those that are most stable; that is, those that are the conjugate bases of strong acids. An identical reactivity order is found for the SN1 reaction because the leaving group is directly involved in the rate-limiting step. Thus, the SN1 reactivity order is
 Note that in the SN1 reaction, which is often carried out under acidic conditions, neutral water is sometimes the leaving group. This occurs, for example, when an alkyl halide is prepared from a tertiary alcohol by reaction with HBr or HCl. As shown in Figure 7.8, the alcohol is first protonated and then spontaneously loses H2O to generate a carbocation, which reacts with halide ion to give the alkyl halide. Knowing that an SN1 reaction is involved in the conversion of alcohols to alkyl halides explains why the reaction works well only for tertiary alcohols. Tertiary alcohols react fastest because they give the most stable carbocation intermediates.
Note that in the SN1 reaction, which is often carried out under acidic conditions, neutral water is sometimes the leaving group. This occurs, for example, when an alkyl halide is prepared from a tertiary alcohol by reaction with HBr or HCl. As shown in Figure 7.8, the alcohol is first protonated and then spontaneously loses H2O to generate a carbocation, which reacts with halide ion to give the alkyl halide. Knowing that an SN1 reaction is involved in the conversion of alcohols to alkyl halides explains why the reaction works well only for tertiary alcohols. Tertiary alcohols react fastest because they give the most stable carbocation intermediates.
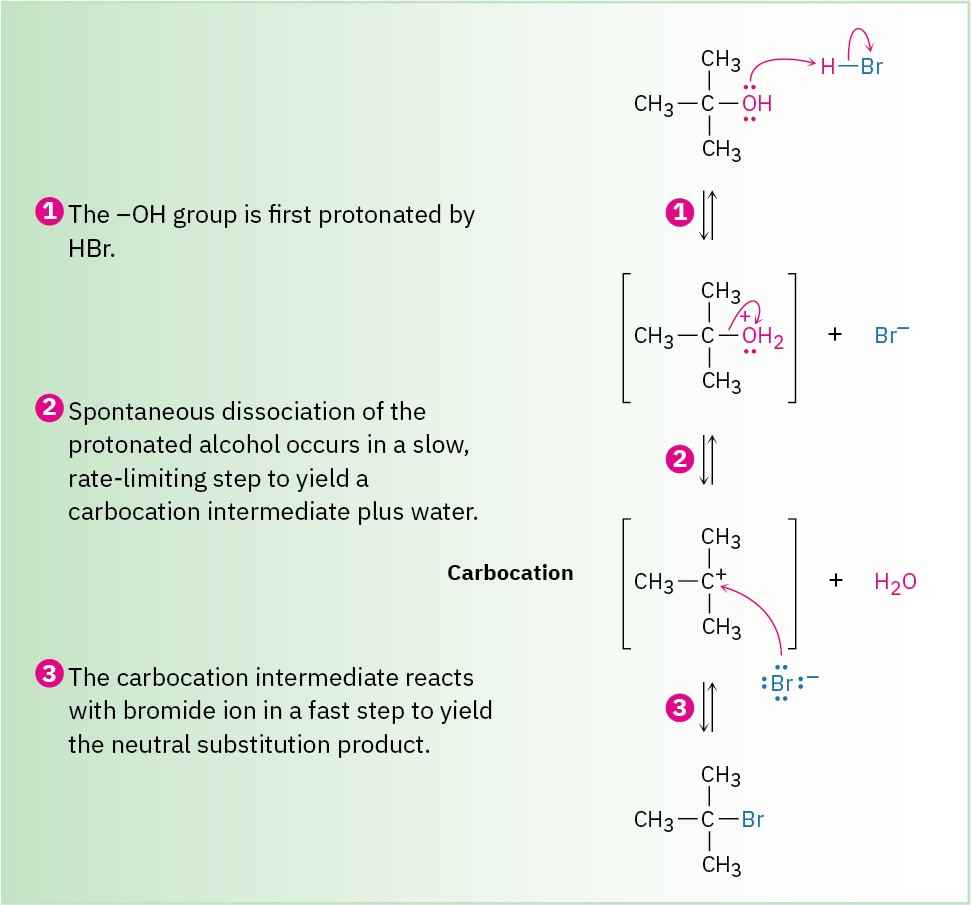
Figure 7.8 MECHANISM: The mechanism of the SN1 reaction of a tertiary alcohol with HBr to yield an alkyl halide. Neutral water is the leaving group (step 2).
The Nucleophile
The nature of the nucleophile plays a major role in the SN2 reaction but does not affect an SN1 reaction. Because the SN1 reaction occurs through a rate-limiting step in which the added nucleophile has no part, the nucleophile can’t affect the reaction rate. The reaction of 2-methyl-2-propanol with HX, for instance, occurs at the same rate regardless of whether X is Cl, Br, or I. Furthermore, neutral nucleophiles are just as effective as negatively charged ones, so SN1 reactions frequently occur under neutral or acidic conditions.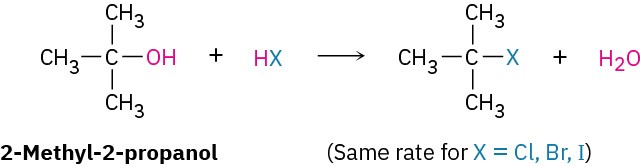
A Summary of SN1 Reaction Characteristics
The effects on SN1 reactions of the four variables—substrate, leaving group, and nucleophile—are summarized in the following statements:
|
Substrate |
The best substrates yield the most stable carbocations. As a result, SN1 reactions are best for tertiary, allylic, and benzylic halides. |
|
Leaving group |
Good leaving groups increase the reaction rate by lowering the energy level of the transition state for carbocation formation. |
|
Nucleophile |
The nucleophile must be nonbasic to prevent a competitive elimination of HX (Section 7.7), but otherwise does not affect the reaction rate. Neutral nucleophiles work well. |

