Chemistry Matters — The Art of Organic Synthesis

Figure 5.17 Vitamin B12 has been synthesized from scratch in the laboratory, but the bacteria growing on sludge from municipal sewage plants do a much better job. (credit: “Aeration and sludge-wasting” by U.S. Department of Agriculture/Flickr, Public Domain)
If you think some of the synthesis problems at the end of this chapter are difficult, try devising a synthesis of vitamin B12 starting only from simple substances you can buy in a chemical catalog. This extraordinary achievement was reported in 1973 as the culmination of a collaborative effort headed by Robert B. Woodward of Harvard University and Albert Eschenmoser of the Swiss Federal Institute of Technology in Zürich. More than 100 graduate students and postdoctoral associates contributed to the work, which took more than a decade to complete.
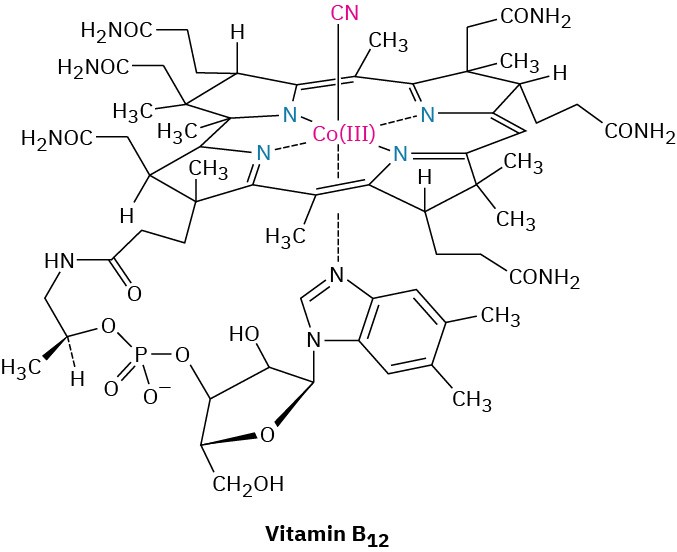
Why put such extraordinary effort into the laboratory synthesis of a molecule so easily obtained from natural sources? There are many reasons. On a basic human level, a chemist might be motivated primarily by the challenge, much as a climber might be challenged by the ascent of a difficult peak. Beyond the pure challenge, the completion of a difficult synthesis is also valuable in that it establishes new standards and raises the field to a new level. If vitamin B12 can be made, then why can’t any molecule found in nature be made?
Indeed, the decades that have passed since the work of Woodward and Eschenmoser have seen the laboratory synthesis of many enormously complex and valuable substances.
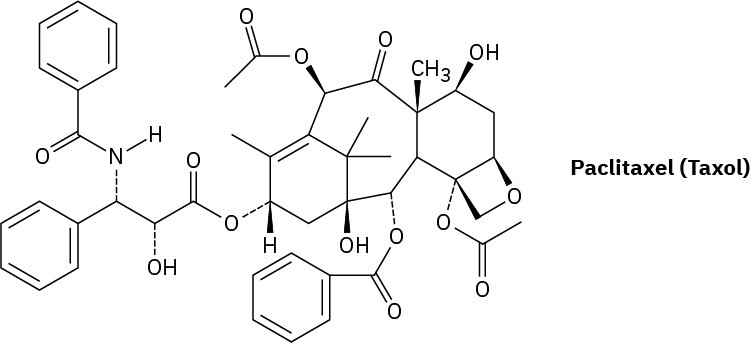
Sometimes these substances—for instance, the anticancer compound paclitaxel, trade named Taxol—are not easily available in nature, so laboratory synthesis is the only method for obtaining larger quantities.
But perhaps the most important reason for undertaking a complex synthesis is that, in so doing, new reactions and new chemistry are discovered. It invariably happens in a complex synthesis that a point is reached at which the planned route fails. At such a time, the only alternatives are either to quit or to devise a way around the difficulty. New reactions and new principles come from such situations, and it is in this way that the science of organic chemistry grows richer. In the synthesis of vitamin B12, for example, unexpected findings emerged that led to the understanding of an entire new class of reactions—the pericyclic reactions. From synthesizing vitamin B12 to understanding pericyclic reactions—no one could have possibly predicted such a link at the beginning of the synthesis, but that is the way of science.

