11.8 Chemistry of Acid Anhydrides
Preparation of Acid Anhydrides
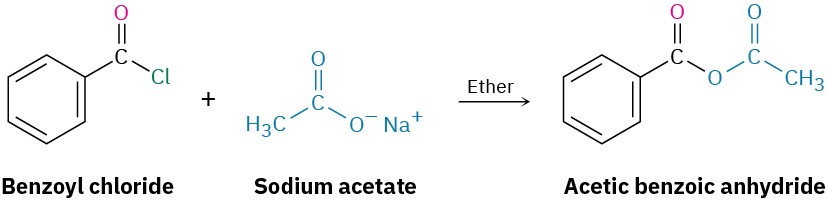
Acid anhydrides are typically prepared by nucleophilic acyl substitution reaction of an acid chloride with a carboxylate anion, as we saw in the previous section. Both symmetrical and unsymmetrical acid anhydrides can be prepared in this way.
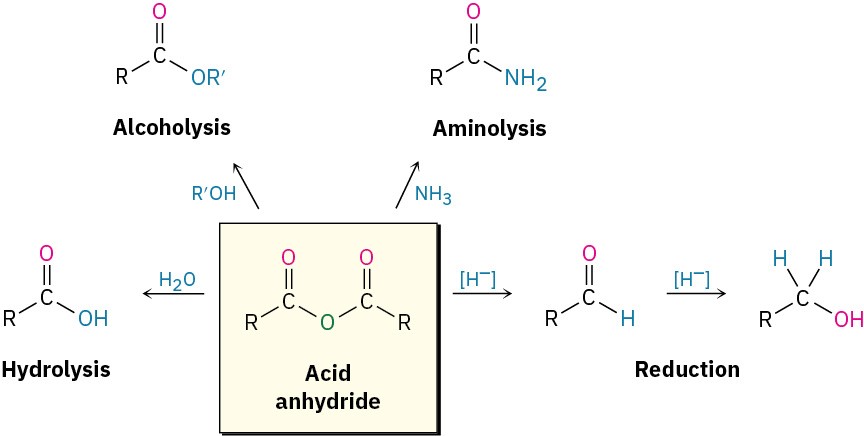
Reactions of Acid Anhydrides
The chemistry of acid anhydrides is similar to that of acid chlorides, although anhydrides react more slowly. Thus, acid anhydrides react with water to form acids, with alcohols to form esters, with amines to form amides, and with LiAlH4 to form primary alcohols. Only the ester- and amide-forming reactions are commonly used, however.
Conversion of Acid Anhydrides into Esters
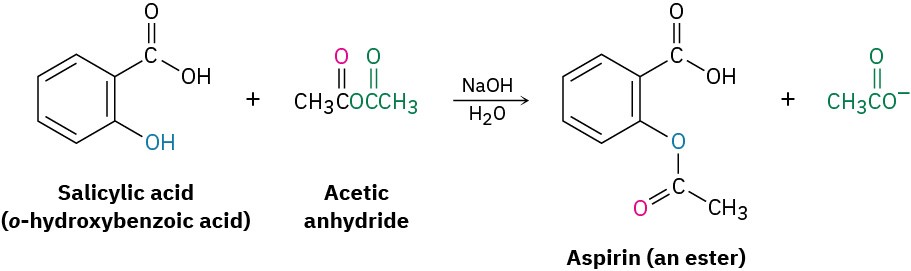
Acetic anhydride is often used to prepare acetate esters from alcohols. For example, aspirin (acetylsalicylic acid) is prepared commercially by the acetylation of o-hydroxybenzoic acid (salicylic acid) with acetic anhydride.
Conversion of Acid Anhydrides into Amides
Acetic anhydride is also commonly used to prepare N-substituted acetamides from amines. For example, acetaminophen, a drug used in over-the-counter analgesics such as Tylenol, is prepared by reaction of p-hydroxyaniline with acetic anhydride. Only the more nucleophilic –NH2 group reacts rather than the less nucleophilic –OH group.
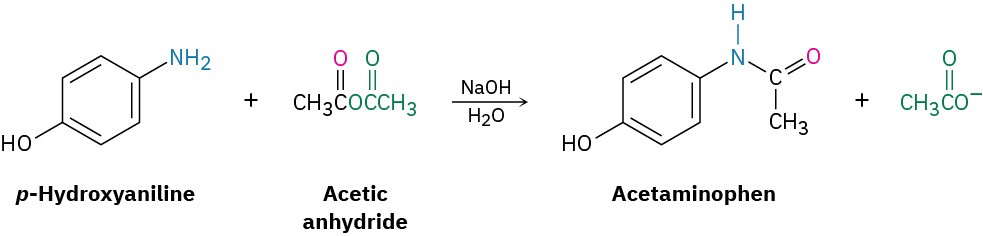 Notice in both of the previous reactions that only “half” of the anhydride molecule is used, while the other half acts as the leaving group during the nucleophilic acyl substitution step and produces acetate ion as a by-product. Thus, anhydrides are inefficient, and acid chlorides are normally preferred for introducing acyl substituents other than acetyl groups.
Notice in both of the previous reactions that only “half” of the anhydride molecule is used, while the other half acts as the leaving group during the nucleophilic acyl substitution step and produces acetate ion as a by-product. Thus, anhydrides are inefficient, and acid chlorides are normally preferred for introducing acyl substituents other than acetyl groups.
Problem 11.22
Write the mechanism of the reaction between p-hydroxyaniline and acetic anhydride to prepare acetaminophen.
Problem 11.23
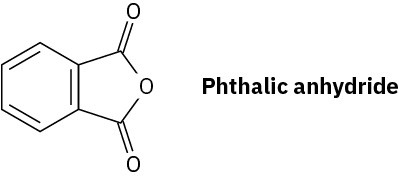
What product would you expect from reaction of one equivalent of methanol with a cyclic anhydride, such as phthalic anhdride (1,2-benzenedicarboxylic anhydride)? What is the fate of the second “half” of the anhydride?